Regardless of their genetic makeup, viruses, which are not living cells, depend on host cells to replicate, express, and assemble their genomes. Instead of cell division, viruses generate new particles by assembling pre-made components, and some even manipulate cellular processes to create a better environment for viral gene expression and evade host defenses. Viral replication is a multi-step process that includes attachment, entry, uncoating, genome replication and expression, assembly, maturation, and release from the host cell. This article will focus on viral genome replication and expression, highlighting the diverse strategies viruses use to control gene expression and ensure their propagation, with a particular emphasis on Where Does Replication Occur within the host cell.
One of the defining characteristics of viruses is their reliance on living cells for replication and propagation. Viruses lack the complete machinery for essential life functions and depend on host cells for energy in the form of nucleoside triphosphates, protein synthesis systems, nucleic acid synthesis components, and structural cell components like lipid membranes. The extent of this dependence varies among virus groups, often correlating with genome complexity. Despite this dependence, viruses exhibit remarkable efficiency and simplicity in genome replication. Viral genomes serve dual roles as mRNA for translation and templates for transcription and replication, containing regulatory RNA elements for these processes. Viral or host proteins and RNA molecules interact with these genomes and cellular pathways, allowing viruses to hijack and customize host cell machinery for their production. This intricate interaction has led to a continuous evolutionary battle between viruses and hosts regarding gene expression and nucleic acid synthesis.
This discussion delves into the strategies of viral genome replication, focusing on how viruses exploit host biology, control gene expression, and ensure their propagation. The viral replication cycle, from entry to progeny virus release, involves initiation of infection, genome replication and expression, and virion egress.
An Overview of Viral Genome Replication Locations
From a viral perspective, the primary goal is to produce progeny and ensure species survival. This is achieved by generating numerous genome copies and packaging them into virions to infect new hosts. Early in infection, viruses must express their genes as functional mRNAs to direct host cell translation machinery to synthesize viral proteins. The path from genome to mRNA varies among viruses, depending on their genome type. Viral genomes differ significantly from cellular genomes, existing as dsDNA, ssDNA, dsRNA, and ssRNA, each with unique transcription modes. Some RNA viruses use their genome directly as mRNA, while others, along with DNA viruses, synthesize mRNA after entering the host cell.
Figure 3.1.
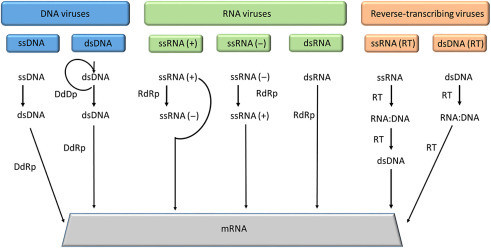 Figure 3.1
Figure 3.1
Summary of replication and transcription modes across different virus classes. Key enzymes involved include DNA-dependent DNA polymerase (DdDp), DNA-dependent RNA polymerase (DdRp), RNA-dependent RNA polymerase (RdRP), and reverse transcriptase (RT). ssRNA(+) serves as mRNA directly.
RNA viruses employ unique replication pathways: RNA-dependent RNA synthesis or, in retroviruses, RNA-dependent DNA synthesis (reverse transcription) followed by DNA replication and transcription. These pathways require enzymes not typically found in host cells, necessitating viral encoding of these enzymes, either expressed early in infection or packaged within virions. It’s important to note that RNA synthesis from an RNA template is also termed “transcription,” differing from the conventional DNA-to-mRNA transcription.
Double-stranded RNA viruses carry their RNA synthesizing enzymes within virions. Once inside the cell, these enzymes transcribe one dsRNA strand into ssRNA(+) within the virion. Released into the cytoplasm, this ssRNA(+) is translated by host machinery and also serves as a template for (−) RNA strand synthesis, which is converted back to dsRNA for packaging. These viruses must synthesize both viral RNA and mRNA, using an “antigenome” or “minus” (−) strand replication intermediate as a template. Single-stranded RNA genomes come in (+) sense (mRNA-like) and (−) antisense (non-mRNA) forms. ssRNA(+) genomes act directly as mRNA and are infectious, immediately translating into proteins, including replication enzymes. Conversely, (−) RNA requires a virus-encoded RNA-replicating enzyme within the virion to transcribe (−) RNA into monocistronic (+) mRNA for host cell translation, and also to synthesize progeny genomes. Retroviruses, another class of (+) RNA viruses, carry an enzyme converting ssRNA(+) to dsDNA upon infection. This viral DNA integrates into the host genome, becoming a provirus, and is transcribed like a cellular gene. Some full-length ssRNA(+) transcripts are packaged into new virions. Maintaining balance between transcription and genome replication is crucial for viral proliferation, achieved through trans-acting proteins, regulatory RNA structures, changes in viral RNA synthesizing enzyme complexes, or a combination of these.
DNA viruses primarily need to produce mRNA. Replication strategies are similar to cellular processes: mRNA transcription from dsDNA and dsDNA replication, usually in the cell nucleus. Many DNA viruses utilize host enzymes, while larger ones encode their own. ssDNA viruses first convert their ssDNA to dsDNA using host enzymes, then transcribe mRNA. Cellular splicing machinery typically processes viral mRNAs. The switch from transcription to replication, or from antigenome production to genomic nucleic acid packaging, is tightly regulated, especially in DNA viruses where genomic DNA replication timing is strictly controlled. Early genes, coding for catalytic and regulatory proteins, are transcribed before viral DNA replication. Late genes, coding for structural components, are transcribed post-DNA replication.
Polymerases and Replication Sites
Viruses depend on polymerases for genome replication and transcription, either encoding their own or utilizing host enzymes. Large DNA viruses like Herpesviridae, Adenoviridae, Poxviridae, and giant viruses encode most of their replication proteins, including those for replication origin recognition, DNA binding, helicases, primases, DNA polymerase, exonucleases, thymidine kinase, and dUTPase. Smaller DNA viruses (Papillomaviridae, Polyomaviridae, Parvoviridae) encode fewer proteins due to genome size limits, instead manipulating cellular activities. Viruses replicating outside the nucleus typically encode their own polymerases.
Four main polymerase types are used by viruses, depending on genome type and replication site (nucleus or cytoplasm): RNA-dependent RNA polymerases (RdRps), RNA-dependent DNA polymerases, DNA-dependent RNA polymerases, and DNA-dependent DNA polymerases. DNA viruses use DNA polymerase for genome replication and DNA-dependent RNA polymerase for mRNA transcription. Both (+) and (−) ssRNA viruses use RdRps. Reverse transcriptase (RT) is used to produce DNA from RNA templates, essential for viruses replicating via RNA intermediates and always virus-encoded.
While high replication fidelity is important, some viral polymerases are less accurate, leading to mutations. DNA viruses have low mutation rates (10−6 to 10−8 mutations per base pair per generation) due to the proofreading ability of their DNA polymerases. RNA viruses (except nidoviruses), lacking proofreading RdRps, have higher mutation rates (10−4 to 10−6). Not all mutations persist; detrimental ones are eliminated, while neutral or silent ones may persist.
Initiation, Elongation, and Termination in Replication
Nucleic acid synthesis by viral polymerases involves initiation, elongation, and termination. Both transcription and mRNA synthesis follow these stages.
Initiation: Polymerase machinery is recruited to the viral promoter, and synthesis starts near the 3′ end of the template. Initiation can be primer-independent (de novo) or primer-dependent. De novo initiation, common in RNA viruses, uses a nucleoside triphosphate as a primer. Primer-dependent initiation requires an oligonucleotide or protein primer. After mRNA transcription initiation, DNA viruses and some dsRNA viruses add a 5′-terminal cap using host enzymes. Most ssRNA(+) viruses encode their own capping enzymes, while some ssRNA(−) viruses’ polymerase caps their mRNA. Negative-sense RNA viruses like influenza viruses use “cap snatching” from host pre-mRNAs.
Elongation: Once replication factors and template are assembled, polymerase synthesizes a complementary strand by adding nucleoside monophosphates to the 3′ end. Poliovirus RdRp, for example, can synthesize the entire genome in one binding event.
Termination: Termination releases the new RNA strand and polymerase from the template. Transcription termination involves secondary structure mechanisms or, in eukaryotes, RNA signals for polyadenylation and termination. Viral mRNA polyadenylation is catalyzed by viral polymerase, unlike host mRNA polyadenylation. In nonsegmented negative RNA viruses, transcription termination of upstream genes is necessary for downstream gene initiation, regulating gene expression within a single promoter framework.
Mechanisms of Genome Replication and Location Specificity
The mechanisms viruses use to replicate and transcribe genomes vary based on genome nature and structure, influencing where does replication occur.
Rolling Circle Replication
Rolling circle replication (RCR) is crucial for circular ssDNA genome replication, such as in ΦX174 phage and geminiviruses. It requires highly processive DNA polymerase with strand displacement ability. After infection, host enzymes create a complementary (−) strand to the viral (+) ssDNA genome, forming a replicative form (RF). A viral endonuclease (Rep protein) nicks the RF’s (+) strand, creating 3′ and 5′ ends. DNA polymerase adds deoxyribonucleotides to the 3′ end, using the (−) strand as a template. The (−) strand revolves, rolling the circle, while DNA synthesis displaces the 5′ end as a tail. Once the (−) strand is fully copied, the (+) strand tail is cut into genome-length units by endonuclease, yielding multiple linear DNA molecules, which are then ligated into circular ssDNA. The RF also serves for mRNA generation for viral protein synthesis. RCR is also implicated in dsDNA virus replication like herpesviruses. Continuous copying of a circular template, followed by discontinuous synthesis on the displaced strand, produces linear dsDNA concatemers (multiple genome copies in a head-to-tail arrangement).
Figure 3.2.
Rolling circle replication mechanism. Continuous copying from a circular template leads to concatemeric DNA, which is then cleaved into unit-length genomes.
Rolling-Hairpin Replication
A variation of RCR, rolling-hairpin replication, is used by linear ssDNA adeno-associated viruses (AAVs). AAVs encode Rep78 protein, similar to RCR initiators, and have inverted terminal repeats (ITRs) at DNA ends, forming hairpin structures. Rep protein interacts with ITRs, initiating replication using host machinery. Rep nicks between the hairpin and coding sequences, creating a 3′-OH primer at the 3′ ITR. DNA synthesis proceeds to the genome end, duplicating the 5′ ITR. Termini refolding regenerates template DNA secondary structures, resulting in a fully replicated genome with original secondary structures.
dsDNA Bidirectional Replication
Bidirectional replication is the classic mode used by eukaryotes and most nuclear dsDNA viruses, including many phages. Replication starts at specific genome sites called origins of replication (ori). Some viruses have multiple ori sites. Replication initiation complexes assemble at ori, followed by topoisomerases unwinding dsDNA and preventing supercoiling. ssDNA-binding proteins keep strands separated, forming a replication fork or bubble. Primase synthesizes RNA primers, and DNA polymerase synthesizes DNA in the 5′ to 3′ direction. Leading strand synthesis is continuous from the primer’s 3′ end. Lagging strand synthesis is discontinuous, producing Okazaki fragments, which are ligated after primer removal by RNase H. This process primarily occurs within the nucleus for viruses utilizing this mechanism, leveraging host cell machinery found there.
dsDNA (RT) Transcription and Replication
Pararetroviruses like Caulimoviridae (circular dsDNA) use an RNA intermediate (pregenomic RNA or pgRNA), similar to retroviruses. Replication has two phases: pgRNA transcription from viral DNA in the nucleus and reverse transcription in the cytoplasm. Unlike retroviruses, viral DNA remains episomal, not integrating into the host genome. Host polymerase transcribes viral DNA into pgRNA, which is transported to the cytoplasm. There, pgRNA is translated into viral proteins, including RT, and also serves as a template for reverse transcription by viral RT. The resulting dsDNA is packaged into virions or returned to the nucleus for further transcription. This dual location of nucleus and cytoplasm is key for pararetrovirus replication.
ssRNA (RT) Replication
Retroviridae family members utilize ssRNA (RT) replication in the cytoplasm post-viral entry. Genomic RNA is reverse transcribed into dsDNA. An RNA:DNA duplex is formed, and RNase H degrades the RNA. A polypurine stretch resists degradation and primes cDNA synthesis. This cDNA integrates into the host genome, becoming a provirus, and undergoes cellular transcription and translation. Integration, facilitated by viral integrase (IN) enzyme recognizing long terminal repeats (LTRs) at linear viral DNA termini, is crucial. Nuclear localization signals assist nuclear migration in some retroviruses. Preintegration complexes enter the nucleus through nuclear pore complexes (NPCs), or during cell division when the nuclear membrane dissolves. Inside the nucleus, viral IN inserts viral DNA into host DNA. LTRs join host DNA in end-processing and end-joining steps, followed by cellular repair. Cellular proteins prevent autointegration. Retroviruses target different integration sites. This process is cytoplasmic initially for reverse transcription, then nuclear for integration and subsequent transcription.
Figure 3.3.
Retroviral DNA integration into the host genome. LTRs are key to the integration process into host DNA.
Positive-Strand RNA Virus Replication
ssRNA(+) viruses replicate in the host cell cytoplasm. ssRNA(+) acts as both replication and transcription template. The 5′ end may be naked, capped, or protein-linked; the 3′ end may be naked or polyadenylated. After viral entry, ssRNA(+) is translated, typically producing a polyprotein encoding replication proteins. Replication forms a dsRNA intermediate, triggering immune responses. To evade this, replication occurs in membrane-associated replication factories in the cytoplasm. This dsRNA is transcribed into genome-length ssRNA(+) for replication or translation. Many ssRNA(+) viruses produce subgenomic RNAs (sgRNAs) for structural or movement proteins, generated via internal initiation, premature termination, or discontinuous RNA synthesis. sgRNAs can regulate translation/replication, act as riboregulators, or support RNA recombination. The cytoplasm is the primary site for all these activities.
Double-Strand RNA Virus Transcription and Replication
dsRNA viral genomes may have naked, capped, or protein-linked 5′ ends. Genomic dsRNA is transcribed into viral mRNA for translation and genome replication. Translation yields replication and encapsidation proteins. dsRNA triggers immune responses, so many dsRNA viruses replicate within icosahedral capsids. RNA polymerases inside the capsid produce mRNA strands extruded from the particle, preventing cytoplasmic dsRNA detection and immune evasion. This capsid-localized replication is a key feature.
Negative-Strand RNA Virus Transcription and Replication
ssRNA(−) viruses (except deltaviruses) replicate in the cytoplasm. Virus-encoded RdRp converts (−) RNA to (+) RNA templates. (+) RNA serves as mRNA for translation and as a template for more (−) RNA strands. RdRp complex handles both replication and transcription. Newly synthesized (−) RNA is encapsidated. Nonsegmented and segmented genome subgroups exist. Segmented genome viruses replicate in the nucleus; RdRp produces monocistronic mRNA from each segment. While transcription of segmented viruses may involve the nucleus, the primary site of replication is the cytoplasm.
dsDNA Template Transcription and Replication
dsDNA viruses replicating in the nucleus or cytoplasm use this template type. Transcription is similar to eukaryotic transcription, in three steps: initiation (transcription initiation complex at promoter), elongation (polymerase recruitment, CTD phosphorylation), and termination (signal recognition, polyadenylation). Replication of dsDNA viruses, whether in the nucleus or cytoplasm, depends on DNA polymerases and follows mechanisms like bidirectional replication or rolling circle replication, with the location being either nuclear or cytoplasmic depending on the virus.
Viral Genome Expression: Tailoring Translation to Viral Needs
Host cell translation machinery starts at the 5′ initiation codon, moves along mRNA, and terminates at a stop codon. Viruses must use this machinery for productive infection, remaining stable and undetected. Viral transcripts often differ from cellular mRNAs, lacking 5′ caps or having structured 5′ leader regions, hindering translation. Cellular mRNAs are mostly monocistronic, while viruses often need to express multiple proteins from their mRNAs. Viruses have evolved mechanisms to customize translation.
DNA viruses in the nucleus often exploit cellular capping machinery. Other strategies include internal initiation of uncapped RNA translation (picornaviruses), cap snatching (segmented negative-strand RNA viruses), and encoding eukaryotic-type capping enzymes.
Poly(A) tails stabilize mRNA in the cytoplasm. Many ssRNA(+) viruses lack poly(A) tails but are still efficiently translated, sometimes using conserved 5′ and 3′ sequences for circularization and translation.
Eukaryotic cells don’t translate polycistronic mRNA into multiple proteins. DNA viruses use splicing. RNA viruses use monocistronic sgRNAs, segmented genomes, or polyprotein synthesis with proteolytic cleavage. These mechanisms have consequences: sgRNA-expressed proteins may separate replication complex components, segmented viruses need correct segment packaging, and polyprotein expression is resource-inefficient. More efficient mechanisms include internal ribosome entry, leaky scanning, ribosome shunting, reinitiation, frameshifting, and stop codon read-through, alongside viral proteins and RNA structures enhancing translation. Regulatory signals in viral mRNAs enable preferential viral gene expression by shutting off host gene expression.
Disruption of Transcription Initiation Complex Assembly
Transcription initiation requires a multiprotein DNA-binding transcription initiation complex. Viruses interfere with host transcription initiation factor loading, shutting down host protein synthesis. Viral mRNA transcripts compete with and preferentially access cellular gene expression machinery.
Figure 3.4.
Viral strategies to downregulate host transcription. Various viral proteins target key host transcription factors like TBP and TFIIH.
Termini Maturation and Modification
mRNA end-processing, including 5′ capping and 3′ poly(A) tailing, is crucial. The 5′ cap is essential for translation initiation and mRNA stability. Cap snatching by ssRNA(−) viruses involves cleaving short sequences from host mRNAs to cap viral mRNAs. Capping can be host- or virus-derived. Recent findings indicate innate immune system recognition of 2′-O-methylation on caps, which cap-stealing mechanisms circumvent.
Figure 3.5.
Cap snatching mechanism. Viruses acquire 5′ caps from host mRNAs to initiate viral mRNA synthesis.
Internal Ribosome Entry
Cap-independent translation initiation uses internal ribosome entry sites (IRES). IRES elements allow ribosome recruitment in the absence of a 5′ cap, especially under conditions repressing cap-dependent synthesis. Cell stress conditions can switch translation from cap-dependent to IRES-driven. Picornaviruses, lacking 5′ caps, benefit from IRES-dependent translation during ER stress response induced by viral infection.
Poly(A) Tailing
3′ poly(A) tailing protects mRNA from degradation and enhances stability. Viral mRNAs can be polyadenylated through stuttering mechanisms involving RNA polymerase slippage over mononucleotide repeats.
Figure 3.6.
Stuttering mechanism for poly(A) tail addition. RNA polymerase slippage leads to poly(A) tail synthesis on viral mRNA.
RNA Editing
RNA editing allows some viruses to produce multiple proteins from a single gene by inserting or deleting bases in mRNA, creating different mRNAs. Cotranscriptional and posttranscriptional editing exist. Ebolaviruses use RNA editing to increase coding capacity and host adaptation.
Alternative Splicing
Alternative splicing, common in cells, enables different proteins from the same gene through alternative splice sites. Regulated by cellular and viral proteins, it increases viral coding capacity, regulates gene expression timing, and is linked to mRNA nuclear export. Some viruses can export unspliced mRNA. Influenza virus NS1 protein can inhibit cellular gene expression by blocking spliceosome recruitment.
Figure 3.7.
Alternative splicing in parvoviruses. Different proteins are generated from the same mRNA through alternative splice site usage.
Suppression of Termination
Stop codon read-through allows production of C-terminally extended polypeptides, often for replicases. Leaky stop codons allow read-through at varying frequencies.
Programmed Ribosomal Frameshifting
Ribosomal frameshifting is a programmed strategy to produce different proteins from overlapping open reading frames. Ribosomes slip and readjust reading frame, enabling more protein coding in small viral genomes.
Figure 3.8.
Ribosomal frameshifting mechanism. -1 frameshifting alters the reading frame, changing the encoded amino acids.
Leaky Scanning and Translation Reinitiation
Leaky scanning bypasses the first start codon, initiating translation at a downstream AUG codon. This can produce different proteins or proteins with different N-termini.
Ribosomal Shunting
Ribosomal shunting involves discontinuous scanning where ribosomes bypass a large leader region and initiate translation downstream. This expands mRNA coding capacity.
Figure 3.9.
Ribosomal shunting mechanism. Ribosomes bypass the first AUG codon to initiate translation at a downstream AUG.
“2A” Oligopeptides and “Stop-Carry On” Recoding
“2A” oligopeptides mediate “stop-carry on” recoding. They cause ribosome skipping of peptide bond formation at the 2A peptide C-terminus, leading to termination and reinitiation on the same codon, producing two proteins from one ORF.
Subversion of Host Gene Expression: Viral Dominance
Viruses maximize genome coding capacity, mimic host mRNA structures, regulate gene expression, and subvert host cell functions for their replication and translation.
Inhibition of Cellular RNA Polymerase
Viruses inhibit host RNA polymerase activity to reduce cellular mRNA transcription. They can block CTD phosphorylation or signal ubiquitination and degradation of RNA polymerase.
Figure 3.10.
Mechanisms of host RNA polymerase inhibition by viruses. Viruses target CTD phosphorylation or induce polymerase degradation.
Disruption of Cellular mRNA Export Pathways
Viruses disrupt cellular mRNA export to favor viral mRNA export. They inhibit nuclear export receptors or nucleoporins, compromising nucleocytoplasmic trafficking of cellular mRNA.
Figure 3.11.
Inhibition of cellular mRNA export by disrupting the nuclear pore complex (NPC). Viruses interfere with NPC function to block host mRNA export.
Decay of Host mRNAs by Viruses
Viruses degrade host mRNAs using viral endonucleases, followed by host exonuclease degradation. This allows preferential viral mRNA translation and helps viral RNAs escape cellular decay machinery detection.
Figure 3.12.
Viral degradation of cellular mRNA. Viral endonucleases cleave host mRNAs, leading to their decay and favoring viral mRNA translation.
Circumvention of Cellular RNA Decay Machinery
Cytoplasmic viruses circumvent cellular mRNA decay machinery to enable virion production. They repress cellular RNA decay factors or degrade them using viral proteases.
Shutoff of Cellular mRNA Translation
Viruses shut off cellular mRNA translation, while continuing to translate viral mRNAs using noncanonical mechanisms. This is achieved by interfering with eukaryotic translation initiation factors.
Figure 3.13.
Viral shutoff of host translation machinery. Viruses target eukaryotic translation initiation factors and PABP to inhibit host translation.
Recruitment of Cellular Hsp70 Chaperones for Viral Protein Folding
Viruses recruit cellular chaperones like Hsp70 for correct viral protein folding, especially important for complex viral proteins and RNA viruses with high mutation rates.
Compromising Cellular Lipid Metabolism
Viruses manipulate cellular lipid metabolism for replication, morphogenesis, and egress. They enhance lipogenesis, impair degradation, and disrupt export to create lipid-rich environments for replication compartments and virion assembly, particularly for enveloped viruses.
Cell Cycle Disruption for Preferential Viral Replication: Timing is Key
Viruses disrupt host cell cycle control to promote viral replication and virion assembly. They encode proteins targeting cell cycle regulators, arresting the cycle at phases favorable for viral replication. This can increase viral gene expression, virion assembly, and delay apoptosis.
Figure 3.14.
Cell cycle dysregulation by viruses. Viruses manipulate cell cycle checkpoints to favor viral replication.
Viruses can initiate the cell cycle, activate transcription factors, and induce quiescent cells to enter S phase to create environments with nucleotides needed for viral replication. Large DNA viruses can cause cell cycle arrest to compete for cellular DNA replication resources. Viral cell cycle manipulation contributes to pathologies like cancer.
Figure 3.15.
Summary of viral strategies for replication and gene expression. A comprehensive overview of viral manipulation of host cell processes.
Virus Genome Replication Complexes: Specialized Replication Sites
Viruses manipulate cellular activities to recruit macromolecules for replication and gene expression to specific cellular locations, forming viroplasms, virus factories, or replication centers. These specialized microenvironments require coordinated control of cellular biosynthesis, cytoskeleton and motor protein alterations, organelle relocalization, and membrane reorganization. Viral factories often sequester mitochondria and chaperones. Nuclear DNA viruses reorganize nuclear components. These compartments provide scaffolds for efficient gene expression and conceal viral genomes and products from immune detection, highlighting specific locations as crucial for successful replication.
In conclusion, viral replication is a complex process with diverse strategies employed by different virus types. The location of replication, whether in the nucleus or cytoplasm, is critically determined by the type of viral genome, the enzymes required, and the need to evade host defenses. Viruses have evolved sophisticated mechanisms to manipulate host cell machinery and create specialized replication sites to ensure their survival and propagation. Understanding where does replication occur is fundamental to comprehending viral pathogenesis and developing antiviral strategies.