1. Introduction
The COVID-19 pandemic spurred significant scientific and media attention, rapidly increasing the demand for new preventative measures and treatments. Drug repurposing became a focal point, with hydroxychloroquine and ivermectin emerging as potential candidates for off-label use. Throughout 2020 and 2021, speculation about ivermectin’s effectiveness against COVID-19 surged across North America, despite limited robust scientific evidence supporting its use [1-3]. As more rigorous research questioned these early claims, several initial studies faced discredit [4, 5].
Ivermectin, an antiparasitic medication, demonstrated early in vitro potential by inhibiting SARS-CoV-2 [15]. Throughout 2020 and 2021, some observational studies, randomized controlled trials, and meta-analyses suggested its efficacy in treating COVID-19 patients [16-25]. However, by late 2021, concerns arose regarding randomization failures and potential fraud within published randomized controlled trials [5]. Consequently, multiple studies faced retraction [17, 20, 25]. A re-analysis of one meta-analysis, excluding studies with high risk of bias, revealed no survival benefit [26].
It’s crucial to note that neither the FDA in the United States nor Health Canada has approved ivermectin for preventing or treating COVID-19. Both regulatory bodies advise against its use based on findings from large randomized controlled trials [27-30]. Despite these official recommendations, ivermectin gained considerable public attention during the pandemic. Controversial scientific papers ignited debates within the scientific community and, through mainstream and social media, influenced public perception. While some US studies have described early trends in ivermectin prescribing [1-3, 31], the broader public discourse and evidence base continued to evolve. Given the substantial media overlap between the US and Canada, understanding the comparative purchasing patterns across these countries is essential. Analyzing how scientific and social discussions impact drug purchasing is vital, particularly when considering the public health risks associated with widely disseminating studies of questionable scientific rigor during a pandemic. Therefore, this study aimed to assess the influence of significant scientific articles, public announcements, and social media posts on ivermectin purchasing patterns in both the US and Canada. This exploration indirectly addresses the question of “Where Can I Buy Ivermectin?” by examining the context surrounding its purchase and demand during a critical period.
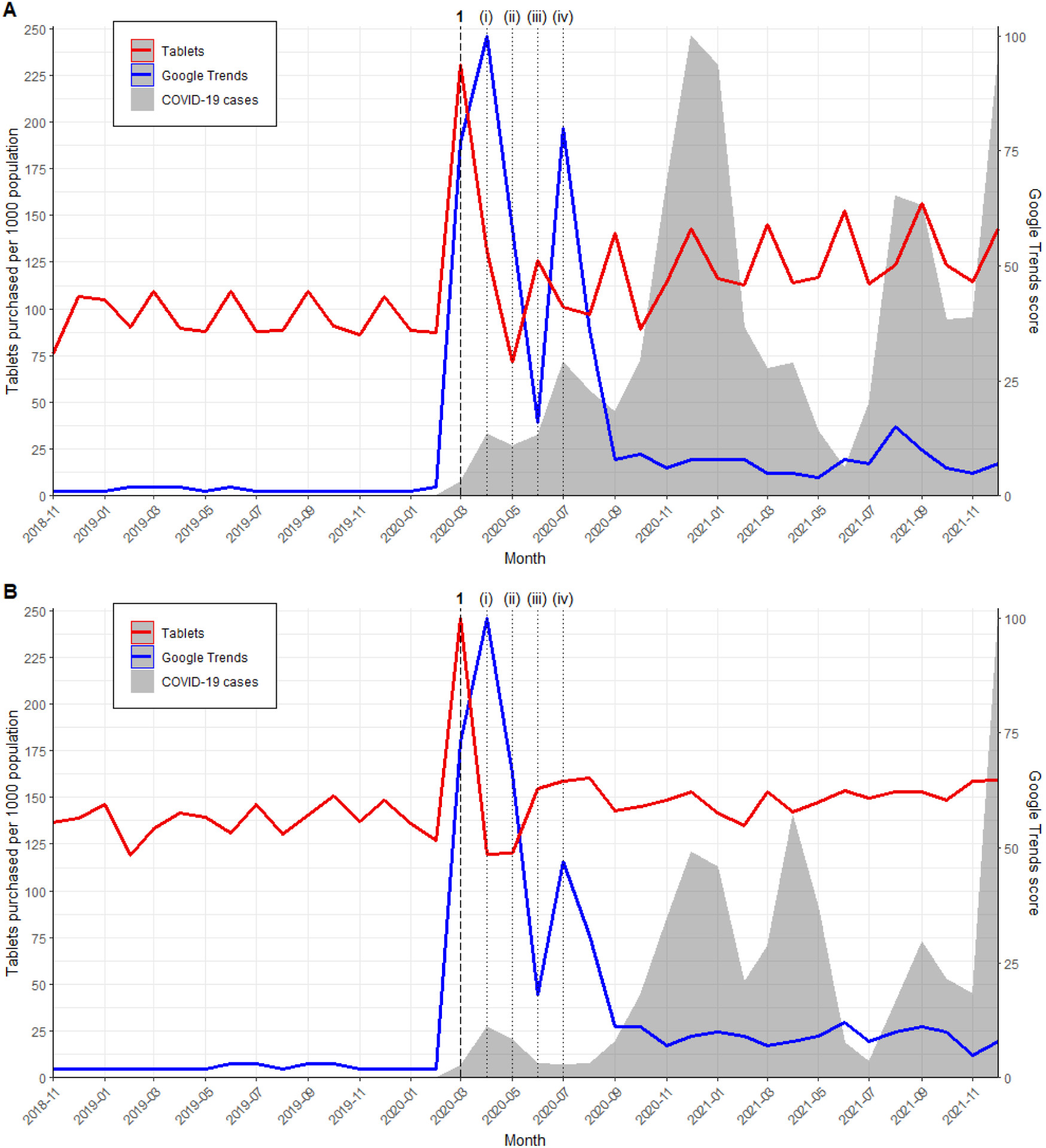 Figure 1.
Figure 1.
2. Methods
2.1. Setting
This research employed a retrospective, population-based time series analysis. We examined outpatient purchases of oral, solid ivermectin units in the US and Canada from February 1, 2016, to December 31, 2021. Canadian ivermectin data collection began in November 2018 due to its later market entry [32]. The University of Pittsburgh Institutional Review Board granted ethical approval (Ref. STUDY21060160).
2.2. Data Sources
Monthly outpatient ivermectin purchase quantities were sourced from IQVIA’s Multinational Integrated Data Analysis (MIDAS) database (Parsippany, NJ, USA). Data was restricted to oral, solid dosage forms, reported as single tablets. MIDAS encompasses comprehensive drug purchasing data for the US and Canada, irrespective of payer, through annual transaction reports from pharmaceutical manufacturers to wholesaler distribution centers [33]. IQVIA annually validates data against alternative sources via a standardized quality assurance program [33]. Data capture rates were 97% in the US and 100% in Canada. Monthly population estimates from the U.S. Census Bureau and Statistics Canada were used to adjust drug purchasing data for population size [34-36]. Canadian population data, available quarterly, was interpolated linearly for monthly estimates.
To gauge public discussion around ivermectin, we utilized Google Trends to extract monthly national web search data for “ivermectin” across all search categories in the US and Canada, from February 2016 to December 2021. Google Trends provides a monthly score from 0 to 100, where 100 signifies peak search interest during the study period [37]. This tool has been previously used to study social responses [9, 38-40] and correlations with prescription patterns [41] during COVID-19. Additionally, monthly COVID-19 case counts for the US and Canada were obtained from the Johns Hopkins Coronavirus Resource Center [42].
2.3. Outcomes
The primary outcome measured was the monthly rate of outpatient ivermectin purchases in the US and Canada, per 1,000 population. These rates were analyzed alongside social discourse trends (Google Trends) and COVID-19 case rates over the same timeframe. (Refer to Supplementary Table S1 for dominant COVID-19 variant details during the study period.)
2.4. Statistical Analysis
Interrupted time series analyses were performed using interventional autoregressive integrated moving average (ARIMA) models to analyze monthly population-adjusted purchasing rates. ARIMA models are suitable for evaluating the impact of population-level health interventions [43]. Interventions were defined as major scientific articles, public announcements, or social media posts concerning ivermectin. An initial list of influential articles, announcements, and posts about ivermectin was compiled from scientific and lay media. Google Trends time series plots were then used to identify peak periods of interest, indicating the most influential interventions. Tables 1 and 2 detail all identified interventions. When multiple interventions occurred close together, the earliest intervention date was selected for model inclusion. Pulse or ramp transfer functions were incorporated to assess temporary or gradual changes in purchasing rates following each intervention. A pulse function models a sudden, temporary change, while a ramp function models a gradual, linearly increasing change over time.
Table 1. Major ivermectin events from the start of the COVID-19 pandemic to December 2021
| Intervention in model | Date in 2020 | Description |
|---|---|---|
| (i) | Not included | March 11 |
| 1 | Pulse | April 3 |
| 2 | Pulse (December to January) | November 13 |
| Intervention in model | Date in 2021 | Description |
| 3 | Ramp (April to August) | April 22 |
| (ii) | Not included | July 28 |
Open in a new tab
Table 2. Key events related to ivermectin and COVID-19 that influenced purchasing trends during the pandemic.
For each ARIMA model, differencing terms and the augmented Dickey-Fuller test were used to achieve and confirm stationarity and seasonality. Seasonality refers to predictable rate fluctuations following a recurring, typically annual pattern. Model parameters were then selected using autocorrelation function (ACF), partial autocorrelation function (PACF), and inverse autocorrelation function (IACF) plots. Final model selection was based on residual autocorrelation plots and the Ljung-Box chi-square test for white noise. Statistical analyses were performed using SAS (Enterprise Guide 7.1, SAS Institute, Cary, NC) with a type 1 error rate of 0.05. Figures are reported from November 2018 onwards (when ivermectin entered the Canadian market) for visualization, but ARIMA models utilized full data from February 2016.
3. Results
3.1. Ivermectin
During the study period (February 2016 to December 2021), US outpatient pharmacies purchased 54,239,000 ivermectin tablets (approximately 0.2 tablets per person), while Canadian counterparts purchased 354,164 tablets (approximately 0.01 tablets per person).
The interrupted time series analysis showed consistent trends across both countries. Following the in vitro study by Caly et al. and the multinational registry analysis by Patel et al. in April 2020, no significant pulse change in monthly purchasing rates was observed in either the US (increase of 0.85 tablets per 1000 population; 95% CI −1.17–2.87; p = 0.41) or Canada (increase of 0.07 tablets per 1000 population; 95% CI −0.03–0.16; p = 0.16). However, after Elgazzar et al.’s pre-print in December 2020 and January 2021, a significant pulse change in ivermectin purchasing was noted in the US (increase of 6.16 tablets per 1000 population; 95% CI 2.66–9.65; p = 0.0006) and Canada (increase of 0.44 tablets per 1000 population; 95% CI 0.36–0.51; p < 0.0001) (Table 3). Purchasing rate trends mirrored Google Trends and COVID-19 case rate trends in both countries. Despite similar intervention responses, population-adjusted ivermectin purchasing rates varied significantly between the US and Canada. The monthly purchase rate peaked at 30 tablets per 1000 population in the US (August 2021), compared to 0.8 tablets per 1000 population in Canada (January 2021) (Figure 2, Supplementary Figure S1).
Table 3. Results of the interventional ARIMA analyses
| Model 3: Ivermectin U.S. | Model 4: Ivermectin Canada |
|---|---|
| ARIMA model | (2,1,0) × (0,1,0)12no intercept |
| Intervention: | Estimate (95% CI) |
| 1 (pulse) | 0.85 (−1.17, 2.87) |
| 2 (pulse) | 6.16 (2.66, 9.65) |
| 3 (ramp) | 1.94 (1.11, 2.75) |
Open in a new tab
Table 3. Statistical outcomes from ARIMA models analyzing the impact of specific events on ivermectin purchasing rates in the US and Canada.
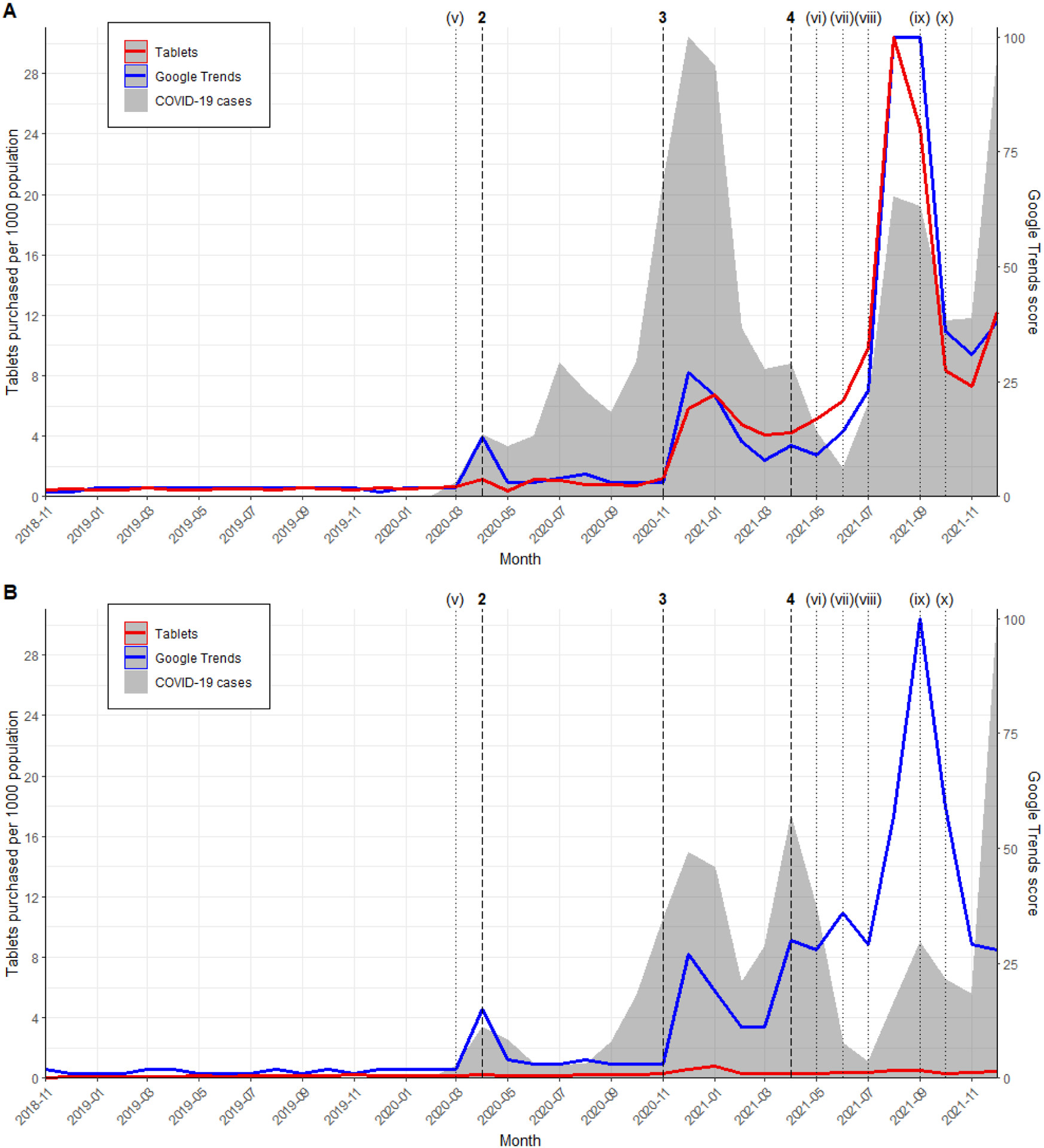 Figure 2.
Figure 2.
4. Discussion
This comprehensive study comparing the US and Canada revealed significantly increased outpatient purchasing rates of ivermectin during the COVID-19 pandemic. Unlike hydroxychloroquine, which saw a short-lived surge, ivermectin experienced a more prolonged sales increase linked to COVID-19 case numbers and publications supporting its effectiveness, even after retractions due to data integrity concerns.
The timeline of scientific evidence dissemination may explain the contrasting purchasing trends between hydroxychloroquine and ivermectin. While initial interest in hydroxychloroquine was potentially fueled by political endorsement, ivermectin gained traction through seemingly supportive randomized controlled trials and meta-analyses [23-25]. The peak in US ivermectin purchases followed meta-analyses incorporating flawed data [23-25], and purchases only declined after concerns about ivermectin trial quality surfaced [5] in September 2021.
The study highlights a critical question for individuals wondering “where can I buy ivermectin?”: availability and access varied significantly between the US and Canada. Despite shared media influence, ivermectin purchasing trends differed notably between the two countries. The US saw a 5-fold purchase increase compared to Canada’s 2-fold increase after the Elgazzar et al. pre-print [17]. This disparity widened further after meta-analyses supporting ivermectin efficacy emerged in April 2021. By August 2021, US purchases reached 30 tablets per 1000 population, while Canada remained at 0.5. This acceleration in the US may be attributed to differing approaches to managing inappropriate prescribing. While US healthcare bodies and medical boards discouraged ivermectin use for COVID-19 [28, 45-48], some state legislatures restricted disciplinary actions against physicians based on COVID-19 prescribing, as seen in Tennessee and North Dakota [49]. Conversely, Canada adopted a more decisive stance. Canadian healthcare bodies and regulators explicitly advised against ivermectin for COVID-19, with Health Canada acting against misleading advertising [30, 50]. Canadian medical, pharmacy, and nursing licensing bodies also strongly opposed ivermectin use, imposing restrictions or suspensions for inappropriate prescribing [51, 52]. These stricter regulatory and professional environments in Canada likely contributed to the lower ivermectin purchasing rates compared to the US.
Most prior studies on hydroxychloroquine and ivermectin trends during COVID-19 focused on the pandemic’s initial year using descriptive methods. Our findings align with previously reported purchasing and dispensing trends in the US [1-3, 31], India [53], Germany [54], and Australia [55]. However, Latin America experienced an early ivermectin surge linked to Patel et al.’s retracted analysis [56], potentially influencing government recommendations in Peru and Bolivia [57]. Ivermectin’s over-the-counter availability [58] and increased production [57] in Latin America might explain this difference.
The ivermectin experience during COVID-19 offers crucial lessons. High demand led to drug shortages [59-61], jeopardizing patients needing ivermectin for approved uses. Safety concerns also arose, with increased ivermectin poisoning cases [46, 62]. Furthermore, reliance on unproven treatments like ivermectin might have deterred individuals from seeking standard care, including vaccination or timely medical intervention. This underscores the importance of consistent, evidence-based public health policies during crises. Improving research reporting, such as enhancing journal data disclosure and accelerating peer review, is crucial for timely, high-quality publications during health emergencies. Media outlets also play a vital role in responsibly covering pre-prints and emphasizing the uncertainties of non-peer-reviewed findings [63].
This study’s strength lies in its use of a large database comprehensively capturing ivermectin purchases across US and Canadian outpatient settings. Employing ARIMA modeling, we analyzed the impact of specific interventions identified through Google Trends, reflecting public interest surges. The extended follow-up period until late 2021 allowed for analyzing multiple interventions. However, limitations exist. Lack of demographic, social, and clinical data prevents exploring trend variations across subgroups. Distinguishing between on-label and off-label use is impossible, though the purchase surges strongly suggest COVID-19-related off-label use. Veterinary ivermectin data was unavailable, likely underestimating total ivermectin use. Numerous concurrent events during the study period complicate isolating individual impacts. For instance, COVID-19 case waves and scientific publications both influenced ivermectin trends. The March 2020 hydroxychloroquine surge might also reflect stockpiling behaviors [54, 55, 64], although the dramatic purchasing increase suggests other factors were at play beyond typical stockpiling [65]. Finally, data limitations to December 2021 preclude analyzing more recent events like nirmatrelvir/ritonavir approval [66, 67] or later trials not supporting ivermectin [68, 69]. Future research could examine recent trends and patient subgroups.
Conclusion
This study demonstrates increased outpatient ivermectin purchases in the US and Canada during the COVID-19 pandemic. The sustained increase in ivermectin purchases correlated with the dissemination of initially promising but ultimately flawed scientific articles. Significant differences emerged between the US and Canada, with the US experiencing much higher ivermectin purchasing rates. The COVID-19 pandemic became an “infodemic,” impacting research, clinical practice, and health policy. This highlights the critical need for evidence-based decision-making and accurate public information dissemination during public health crises.
Supplementary Material
NIHMS1893789-supplement-Supplementary_Material.docx
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The opinions expressed are those of the authors and do not represent those of the U.S. Department of Veterans Affairs, the U.S. government, or IQVIA or any of its affiliated entities. The statements, findings, conclusions, views, and opinions contained and expressed in this study are based in part on data obtained under license from IQVIA: MIDAS February 2016 to December 2021, IQVIA, Inc. The study sponsor had no role in the design or conduct of the study; data collection, management, analysis, or interpretation of the data; preparation, review, or appraisal of the manuscript; or decision to submit the manuscript for publication. The authors would like to acknowledge Daniel McCormack who supported some elements of the data analysis.
Funding
Research reported was support by the Agency for Healthcare Research and Quality under Grant R01HS027985 (Principal Investigator: KJS).
Footnotes
Transparency declarations
None to declare.
References
Associated Data
Supplementary Materials
Supplementary Material

