Dissolved oxygen (DO) is a crucial parameter in water quality assessment. It refers to the level of free, non-compound oxygen present in water or other liquids. This oxygen is essential for aquatic organisms, playing a vital role in the health of rivers, lakes, and oceans. While water molecules themselves contain oxygen, this is not the form that aquatic life can use. Instead, they depend on the small amount of oxygen that is dissolved within the water.
Oxygen enters water bodies primarily from two sources: the atmosphere and groundwater discharge. Atmospheric oxygen dissolves into surface water, and in regions where groundwater significantly contributes to streamflow, oxygen-rich groundwater also becomes a source. This dissolved oxygen is what fish, zooplankton, and other aquatic creatures “breathe” to survive.
 USGS scientist taking a dissolved oxygen reading in a small stream.
USGS scientist taking a dissolved oxygen reading in a small stream.
USGS scientist measuring dissolved oxygen levels in a stream to assess water quality.
The Link Between Dissolved Oxygen and Water Quality
The amount of dissolved oxygen in water is a strong indicator of its quality. Water bodies with rapid movement, like mountain streams and large rivers, typically have higher DO concentrations due to greater aeration. Conversely, stagnant waters tend to have lower levels. Furthermore, the presence of bacteria in water can significantly impact DO levels. As bacteria decompose organic matter, they consume oxygen in the process. An excess of organic material in water bodies, often due to pollution from sources like agricultural runoff or sewage, can lead to a surge in bacterial activity. This, in turn, can deplete dissolved oxygen, creating a condition known as eutrophication.
Eutrophication results in oxygen-deficient environments that can be detrimental, even lethal, to aquatic life. In such conditions, a lake or river can essentially “die” as it becomes unable to support a healthy ecosystem. Aquatic organisms struggle to survive in stagnant, organically rich water, particularly during summer months when dissolved oxygen levels naturally decrease due to the inverse relationship between water temperature and DO concentration. Surface waters (epilimnion) may become too warm, while bottom waters (hypolimnion) suffer from oxygen scarcity. Hot, calm weather can exacerbate this issue, sometimes leading to large-scale fish kills in affected lakes.
 A eutrophic lake, with excess algal growth
A eutrophic lake, with excess algal growth
Eutrophic lake showing algal bloom, an indicator of low dissolved oxygen conditions.
Temperature’s Influence on Dissolved Oxygen
Water temperature plays a critical role in determining dissolved oxygen concentrations. Colder water has a greater capacity to hold dissolved oxygen compared to warmer water. This principle leads to both seasonal and daily cycles in DO levels in surface waters. During winter and early spring, when water temperatures are low, dissolved oxygen concentrations are generally high. Conversely, in summer and fall, higher water temperatures often result in lower DO levels.
The relationship between dissolved oxygen and temperature is primarily mediated by photosynthesis and atmospheric exchange. Photosynthesis by aquatic plants and algae produces oxygen, but this process is influenced by water clarity and sunlight intensity. Oxygen exchange with the atmosphere also slows down as water temperature increases.
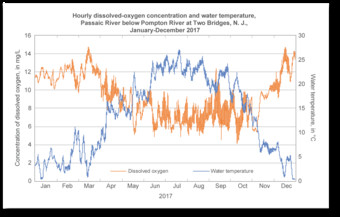 Dissolved oxygen and water temperature for Passaic River below Pompton River at Two Bridges, N. J., 2017
Dissolved oxygen and water temperature for Passaic River below Pompton River at Two Bridges, N. J., 2017
Graph illustrating the inverse relationship between water temperature and dissolved oxygen concentration.
Hypoxia and the Formation of “Dead Zones”
A severe consequence of low dissolved oxygen is hypoxia, which can lead to the formation of “dead zones” in aquatic environments. A prominent example is the Gulf of Mexico “dead zone” near the Mississippi River Delta. This zone develops seasonally due to nutrient-rich discharge from the Mississippi and Atchafalaya Rivers. These nutrients, primarily nitrogen and phosphorus, fuel algal blooms. When these algal blooms die and decompose, bacteria consume vast amounts of oxygen, leading to severely depleted oxygen levels in bottom waters.
When dissolved oxygen concentrations fall below 2 milligrams per liter (mg/L), the water is classified as hypoxic. Such low oxygen levels are insufficient to support most marine life, leading to the displacement or death of organisms that cannot escape the area. The Gulf of Mexico dead zone, situated in a productive fishing area, poses significant economic and environmental challenges to both commercial and recreational fishing industries.
Measuring Dissolved Oxygen Levels
Measuring dissolved oxygen is essential for monitoring water quality and the health of aquatic ecosystems. Both field and laboratory instruments are used for this purpose. Modern DO meters are typically electronic and portable, utilizing a probe that is submerged in the water to take readings. Since dissolved oxygen is temperature-dependent, accurate measurements require proper calibration of the meter, taking temperature into account. Regular monitoring of dissolved oxygen levels helps in understanding the health of water bodies and identifying potential threats to aquatic life.
 A submersible meter to measure multiple water-quality parameters in real time.
A submersible meter to measure multiple water-quality parameters in real time.
Multi-parameter water quality monitor used for real-time dissolved oxygen measurement.
Understanding dissolved oxygen is crucial for appreciating the delicate balance of aquatic ecosystems and the importance of protecting water quality for the life it supports. By monitoring and managing factors that affect dissolved oxygen, we can contribute to healthier rivers, lakes, and oceans for future generations.

