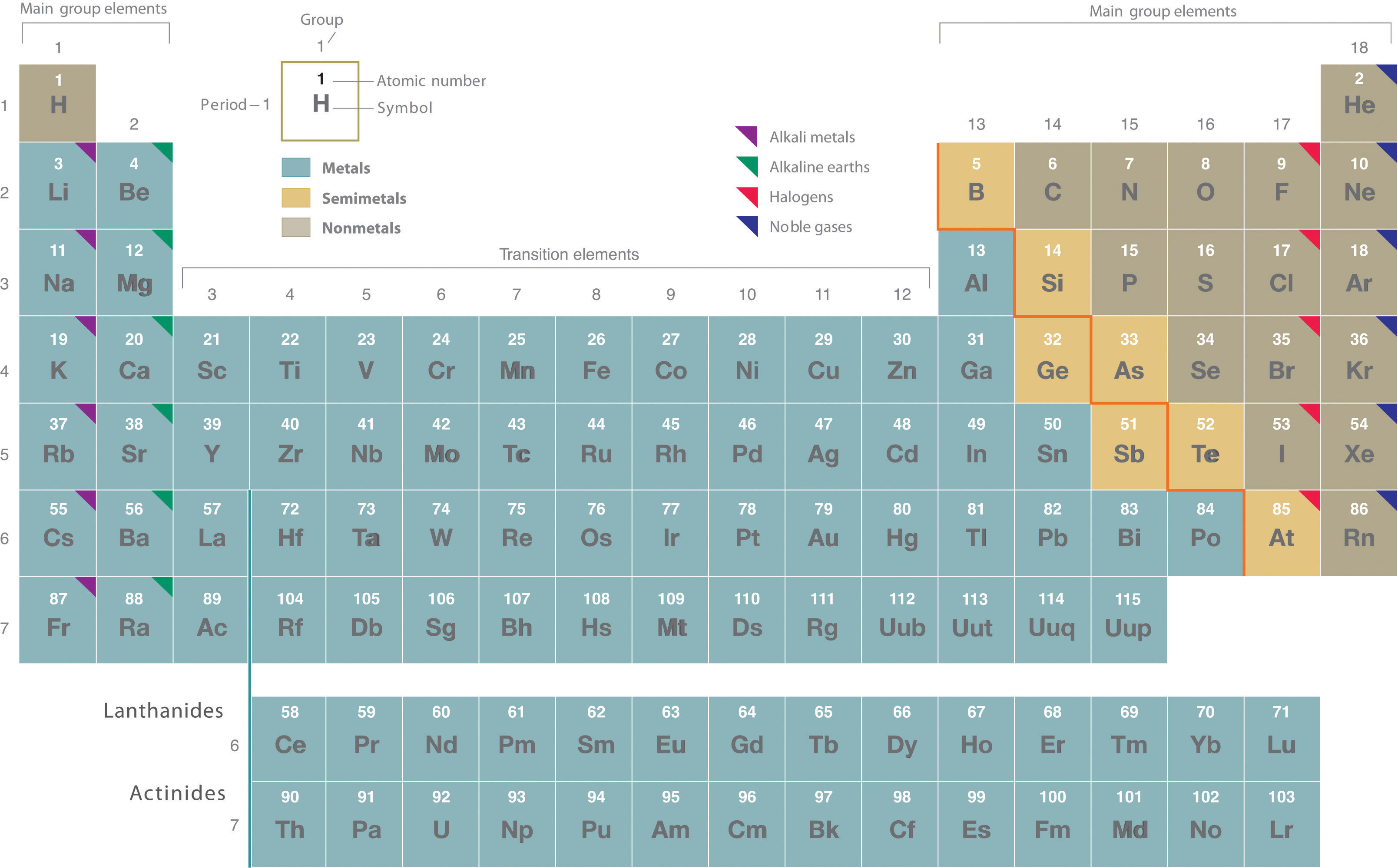
The periodic table is an indispensable tool in chemistry, organizing all known elements in a way that reveals trends in their properties. One of the most fundamental distinctions within this table is the categorization of elements into metals, nonmetals, and semimetals. Understanding Where Are Metals Located On The Periodic Table is key to grasping basic chemical properties and predicting element behavior. This article will explore the location of metals on the periodic table, their defining characteristics, and the groups they predominantly occupy.
Decoding the Periodic Table’s Structure
The periodic table isn’t just a random arrangement; it’s a structured chart based on the atomic number of elements, which is the number of protons in an atom’s nucleus. Elements are arranged in ascending order of their atomic numbers, from left to right and top to bottom. This arrangement creates a table with horizontal rows called periods and vertical columns known as groups. Periods are numbered 1 through 7, while groups are numbered 1 to 18.
 Periodic Table Showing the Elements in Order of Increasing Z
Periodic Table Showing the Elements in Order of Increasing Z
Figure 1: The periodic table, illustrating the organization of elements and the general location of metals on the left side and transitioning towards nonmetals on the right.
This structure is crucial because elements within the same group share similar chemical properties. However, for the purpose of locating metals, a broader division is even more informative: the classification into metals, nonmetals, and semimetals.
The Location of Metals, Nonmetals, and Semimetals: A Periodic Trend
A noticeable feature of the periodic table is the heavy, zigzag diagonal line that steps down and to the right, starting between Boron (B) and Aluminum (Al) and continuing towards Polonium (Po) and Astatine (At). This line acts as a visual divider, separating the periodic table into two primary regions based on elemental properties:
Metals: Predominantly on the Left and Center
Metals are predominantly situated to the left and below this diagonal line on the periodic table. This vast area encompasses the majority of the elements. From Group 1 (Alkali Metals) and Group 2 (Alkaline Earth Metals) on the far left, extending through the transition metals in the center (Groups 3-12), and including the lanthanides and actinides located below the main body of the table, metals dominate the periodic landscape.
Nonmetals: Occupying the Upper Right
In contrast to metals, nonmetals are found in the upper right portion of the periodic table, above and to the right of the zigzag line. This region includes elements like carbon, nitrogen, oxygen, halogens (Group 17), and noble gases (Group 18).
Semimetals (Metalloids): The Dividing Line
Elements that border the zigzag line, such as boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te), are known as semimetals or metalloids. These elements exhibit properties that are intermediate between those of metals and nonmetals, hence their position on the dividing line.
Defining Properties of Metals
The location of metals on the periodic table is directly linked to their characteristic properties. Metals are well-known for:
- High Electrical and Thermal Conductivity: Metals are excellent conductors of both electricity and heat. This is due to the nature of metallic bonding, which allows electrons to move freely.
- Luster (Shiny Appearance): Metals typically have a shiny or lustrous surface when polished.
- Malleability: Metals are malleable, meaning they can be hammered or pressed into thin sheets without breaking. Think of aluminum foil or gold leaf.
- Ductility: Metals are ductile, meaning they can be drawn into wires. Copper, used extensively in electrical wiring, is a prime example.
- Solid State at Room Temperature (Except Mercury): Most metals are solid at room temperature. Mercury is the notable exception, being a liquid metal.
Key Metal Groups in the Periodic Table
Certain groups within the metallic region of the periodic table are particularly important and have specific names:
Alkali Metals (Group 1)
Located in the far left column (Group 1), alkali metals (Lithium, Sodium, Potassium, Rubidium, Cesium, Francium) are highly reactive metals. They readily lose one electron to form positive ions and react vigorously with water and halogens.
Alkaline Earth Metals (Group 2)
Group 2, the alkaline earth metals (Beryllium, Magnesium, Calcium, Strontium, Barium, Radium), are also reactive, though less so than alkali metals. They typically lose two electrons to form positive ions and are essential in various biological and geological processes.
Transition Metals (Groups 3-12)
Occupying the central block of the periodic table, transition metals are characterized by their variable oxidation states and ability to form colorful compounds. They are crucial in many industrial applications and include familiar metals like iron, copper, gold, and silver.
Lanthanides and Actinides
Positioned below the main body of the periodic table, the lanthanides and actinides are also metals. Lanthanides, also known as rare earth metals, are used in various technologies. Actinides are all radioactive, with some, like uranium and plutonium, being important in nuclear energy.
Conclusion: Metals Dominate the Periodic Table’s Left
In summary, metals are predominantly located on the left side and center of the periodic table, below and to the left of the zigzag line. This location is a direct reflection of their shared properties: conductivity, luster, malleability, and ductility. Understanding the location of metals within the periodic table is fundamental to predicting their behavior and appreciating their diverse roles in chemistry and beyond. The periodic table, therefore, serves as a map guiding us through the metallic realm and the properties that define it.
