Acquired immunodeficiency syndrome (AIDS) is a devastating disease caused by two lentiviruses: human immunodeficiency virus type 1 (HIV-1) and human immunodeficiency virus type 2 (HIV-2). The emergence of AIDS in the early 1980s marked the beginning of a global health crisis, prompting intense scientific inquiry into the origins of this novel disease. Understanding Where Did Aids Originate From is not just a matter of historical curiosity; it is crucial for preventing future pandemics and appreciating the complex interplay between viruses and their hosts.
This article delves into the scientific journey to uncover the origins of AIDS, tracing back to its roots in non-human primates and exploring the evolutionary pathways that led to the global pandemic we know today. We will examine the evidence pointing to the zoonotic origins of HIV, the role of simian immunodeficiency viruses (SIVs), and the specific events that facilitated the jump of these viruses from animals to humans.
The Initial Recognition of AIDS and the Discovery of HIV
In 1981, the medical community began to notice a disturbing trend: young, previously healthy homosexual men in the United States were developing rare opportunistic infections and cancers, conditions typically associated with compromised immune systems (CDC 1981; Greene 2007). This cluster of unusual illnesses was soon recognized as a new syndrome, initially termed Gay-Related Immune Deficiency (GRID), but later renamed Acquired Immunodeficiency Syndrome (AIDS).
The search for the causative agent of AIDS intensified, and in 1983, scientists successfully identified a novel retrovirus as the culprit (Barre-Sinoussi et al. 1983). This virus, initially known as lymphadenopathy-associated virus (LAV) and human T-cell lymphotropic virus type III (HTLV-III), was eventually unified under the name human immunodeficiency virus type 1 (HIV-1) (Gallo et al. 1984; Popovic et al. 1984). HIV-1 spreads through sexual contact, blood-to-blood contact, and from mother to child (Hladik and McElrath 2008; Cohen et al. 2011), with the majority of infections occurring through mucosal surfaces during sexual activity (Hladik and McElrath 2008; Cohen et al. 2011).
The impact of HIV-1 has been catastrophic. The pandemic strain, known as group M, has infected an estimated 60 million people globally, resulting in over 25 million deaths (Merson et al. 2008). Developing countries, particularly in sub-Saharan Africa, have borne the brunt of the epidemic (http://www.unaids.org/). While antiretroviral therapy has significantly reduced AIDS-related mortality, access to treatment remains uneven, and a cure or effective vaccine is still elusive (Barouch 2008; Richman et al. 2009). AIDS remains a significant global health challenge.
The Discovery of HIV-2 and Simian Immunodeficiency Viruses (SIVs)
The discovery of HIV-1 sparked intense research into its origins. A crucial breakthrough came in 1986 with the identification of a second, related virus causing AIDS in West Africa (Clavel et al. 1986). This new virus, named human immunodeficiency virus type 2 (HIV-2), was genetically distinct from HIV-1 but surprisingly similar to a simian virus causing immunodeficiency in captive macaques (Chakrabarti et al. 1987; Guyader et al. 1987).
This discovery led to the identification of a family of viruses collectively known as simian immunodeficiency viruses (SIVs), found in various non-human primates across sub-Saharan Africa, including African green monkeys, sooty mangabeys, mandrills, and chimpanzees (Fig. 1). Intriguingly, these SIVs appeared to be largely non-pathogenic in their natural primate hosts, despite their close evolutionary relationship to the AIDS-causing viruses in humans and macaques (Fig. 2). The close genetic similarities between HIV-1 and SIVcpz (simian immunodeficiency virus of chimpanzees), and between HIV-2 and SIVsmm (simian immunodeficiency virus of sooty mangabeys), provided the first compelling evidence that AIDS in both humans and macaques was a result of cross-species transmission of lentiviruses from different primate species (Sharp et al. 1994; Hahn et al. 2000).
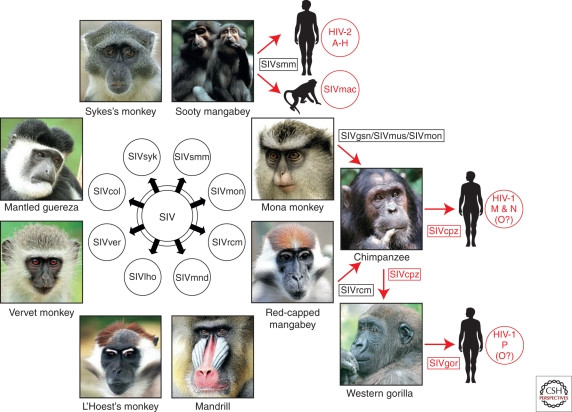 Origins of human AIDS viruses
Origins of human AIDS viruses
Figure 1. Origins of human AIDS viruses. Old World monkeys in Africa carry diverse simian immunodeficiency viruses (SIVs). Cross-species transmissions of certain SIVs to great apes and humans have resulted in new pathogens, highlighted in red. For example, SIVsmm from sooty mangabeys and SIVcpz from chimpanzees are the origins of HIV-2 and HIV-1 respectively.
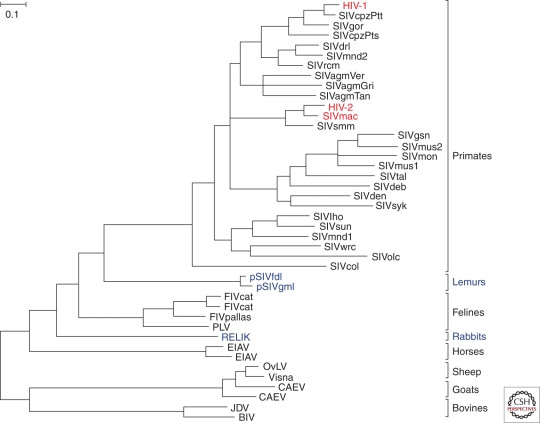 Phylogeny of lentiviruses
Phylogeny of lentiviruses
Figure 2. Evolutionary relationships of lentiviruses. This phylogenetic tree shows the connections between different lentiviruses based on their genetic sequences. HIV-1, HIV-2, and SIVmac, highlighted in red, are closely related to simian viruses, indicating their origin from animal sources.
Primate Lentiviruses: The Ancestors of HIV
Lentiviruses are a group of retroviruses that cause chronic, persistent infections in various mammals. Most lentiviruses are transmitted horizontally between individuals, but some have become “endogenous,” meaning they have integrated into the host’s genome and can be passed down through generations (Fig. 2). These endogenous lentiviruses, or “viral fossils,” like RELIK in rabbits and pSIVgml/pSIVfdl in lemurs, provide a deep-time perspective on lentivirus evolution (Katzourakis et al. 2007; van der Loo et al. 2009; Gifford et al. 2008; Gilbert et al. 2009). They suggest that primate lentiviruses have a much longer evolutionary history than initially estimated based on extant SIV sequences alone (Sharp et al. 2000; Holmes 2003; Wertheim and Worobey 2009).
SIV infections are primarily found in African monkeys and apes, suggesting that primate lentiviruses likely originated in Africa after the evolutionary split between African and Asian Old World monkeys around 6-10 million years ago (Fabre et al. 2009). While sampling of Asian and New World primates is ongoing, current evidence points to Africa as the epicenter of primate lentivirus evolution.
Over 40 different primate species are known to be infected with SIVs (Klatt et al. 2011). While most SIV transmission occurs within the same species, cross-species transmissions are not uncommon. These can range from isolated, “dead-end” infections to the establishment of new SIV lineages capable of further spread (Jin et al. 1994b; van Rensburg et al. 1998; Van Heuverswyn et al. 2006). Recombination between different SIV strains in co-infected animals has also led to the emergence of novel viral variants (Jin et al. 1994a; Souquiere et al. 2001; Aghokeng et al. 2007). The frequency and impact of these cross-species transfers, particularly in relation to the emergence of AIDS, are critical questions in understanding the origins of the pandemic.
SIVcpz: The Chimpanzee Origin of HIV-1
Among the diverse SIVs, SIVcpz, found in chimpanzees, holds particular significance due to its close genetic relationship to HIV-1 (Fig. 2). Early studies on SIVcpz were hampered by the endangered status of chimpanzees and the low prevalence of infection in captive populations (Sharp et al. 2005). However, the development of non-invasive diagnostic methods to detect SIVcpz in chimpanzee fecal and urine samples revolutionized research (Santiago et al. 2003; Keele et al. 2006).
These methods enabled comprehensive surveys of wild chimpanzee populations across central Africa. Chimpanzees are divided into two species: common chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). Common chimpanzees are further classified into subspecies: western (P. t. verus), Nigeria-Cameroonian (P. t. ellioti), central (P. t. troglodytes), and eastern (P. t. schweinfurthii) chimpanzees (Groves 2001; Gagneux et al. 1999).
Surveys revealed that SIVcpz is found in central and eastern chimpanzees, but not in western, Nigeria-Cameroonian chimpanzees, or bonobos (Fig. 3A). Prevalence rates in infected chimpanzee communities vary widely, from 30-50% in some to very low or absent in others (Santiago et al. 2002, 2003; Worobey et al. 2004; Keele et al. 2006; Van Heuverswyn et al. 2007; Li et al. 2010; Rudicell et al. 2010). This patchy distribution contrasts with other SIVs that are more widespread and prevalent in their monkey hosts (Phillips-Conroy et al. 1994; Santiago et al. 2005).
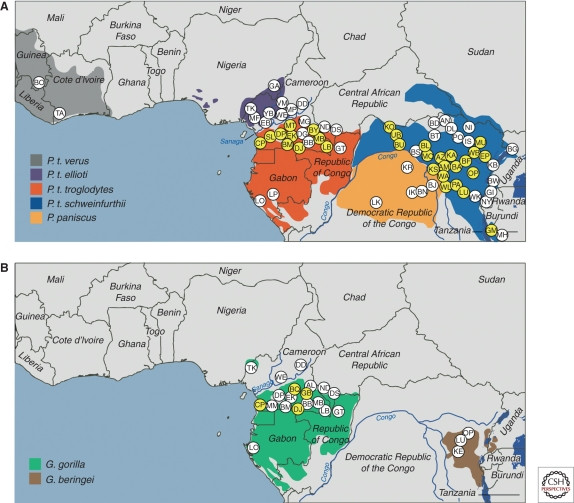 Geographic distribution of SIVcpz and SIVgor
Geographic distribution of SIVcpz and SIVgor
Figure 3. Distribution of SIV infections in African apes. (A) SIVcpz is found in central and eastern chimpanzee subspecies but absent in western and Nigeria-Cameroonian chimpanzees and bonobos. (B) SIVgor is found in western lowland gorillas. Yellow areas indicate sites with detected SIV infections.
Phylogenetic analysis showed that SIVcpz is a mosaic virus, resulting from recombination between two monkey SIV lineages (Bailes et al. 2003). Part of the SIVcpz genome is related to SIVrcm from red-capped mangabeys, while other parts are related to SIVs from Cercopithecus monkeys like greater spot-nosed, mustached, and mona monkeys (Bailes et al. 2003). Chimpanzee hunting behavior, which includes preying on monkeys (Goodall 1986), likely facilitated these cross-species transmissions and the emergence of SIVcpz in central chimpanzees, subsequently spreading to eastern chimpanzees.
SIVcpz Infection in Chimpanzees: Not Always Harmless
Initially, SIVcpz was considered non-pathogenic in chimpanzees, similar to many other SIVs in their natural hosts. This was based on limited observations of captive infected apes (Heeney et al. 2006; Paiardini et al. 2009). However, the sporadic prevalence of SIVcpz and its relatively recent origin hinted at a potentially different natural history compared to other SIVs.
Long-term studies in Gombe National Park, Tanzania, where chimpanzees are habituated and closely observed, revealed a different picture (Santiago et al. 2002; Pusey et al. 2007). These studies showed that SIVcpz is indeed pathogenic in chimpanzees. Infected chimpanzees had a significantly higher mortality rate (10- to 16-fold increased risk of death) compared to uninfected individuals (Keele et al. 2009). SIVcpz infection also negatively impacted female reproduction and infant survival. Postmortem analyses revealed CD4+ T-cell depletion in infected chimpanzees, and one case showed histopathological signs of AIDS (Keele et al. 2009).
These findings, confirmed by subsequent studies (Etienne et al. 2011), demonstrate that SIVcpz can have substantial negative effects on the health, reproduction, and lifespan of wild chimpanzees. The Kalande community in Gombe, with high SIVcpz prevalence, even experienced a population decline (Rudicell et al. 2010), further emphasizing the pathogenic potential of SIVcpz.
SIVgor: A Gorillan Cousin of HIV-1
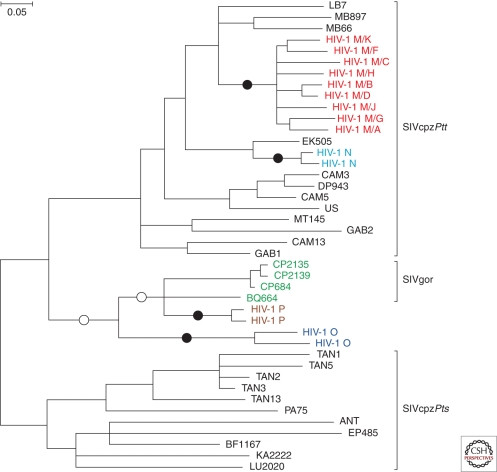
Non-invasive testing also led to the unexpected discovery of a new SIV lineage in gorillas, termed SIVgor (Van Heuverswyn et al. 2006, Fig. 4). SIVgor was found in western lowland gorillas (Gorilla gorilla gorilla) in southern Cameroon and is closely related to SIVcpz strains from central chimpanzees, particularly P. t. troglodytes. Phylogenetic analysis suggests that SIVgor resulted from a single cross-species transmission event from chimpanzees to gorillas, estimated to have occurred 100-200 years ago (Takehisa et al. 2009).
 HIV-1 origins and related SIVs
HIV-1 origins and related SIVs
Figure 4. Phylogenetic tree showing HIV-1, SIVcpz, and SIVgor relationships. All HIV-1 groups and SIVgor cluster with SIVcpz from central chimpanzees, indicating chimpanzees as the source. Independent cross-species transmissions to humans (black circles) and gorillas (white circles) are highlighted.
SIVgor appears to be less common in gorillas than SIVcpz in chimpanzees, with lower prevalence rates and fewer infected communities identified (Neel et al. 2010, Fig. 3B). How gorillas, which are herbivores, acquired SIVgor remains a mystery. However, gorillas and chimpanzees share habitats, suggesting potential encounters that could have facilitated transmission. The pathogenicity of SIVgor in gorillas is currently unknown due to limited research opportunities.
Tracing the Origins of HIV-1 Groups
HIV-1 is not a single virus but comprises four distinct groups: M, N, O, and P. Each group represents a separate cross-species transmission event from chimpanzees to humans. Group M is responsible for the global pandemic, while groups N, O, and P are far less prevalent and geographically restricted.
Phylogenetic analysis firmly places the origin of all four HIV-1 groups within the SIVcpzPtt lineage found in central chimpanzees (Fig. 4). Groups M and N are most closely related to SIVcpzPtt strains from southern Cameroon, pinpointing this region as the likely origin of these human viruses (Keele et al. 2006; Van Heuverswyn et al. 2007). Specifically, group N likely emerged near the Dja Forest, and the pandemic group M originated near the Boumba-Ngoko-Sangha rivers region in southeastern Cameroon. While group P also likely originated from gorillas, the exact location is still being investigated due to limited SIVgor samples. The origin of group O remains less clear, with no currently identified ape virus being a direct ancestor, although it is also likely of chimpanzee or gorilla origin from west-central Africa.
The exact circumstances of these chimpanzee-to-human transmissions are unknown, but bushmeat hunting, which involves exposure to infected ape blood and body fluids, is considered the most probable route (Peeters et al. 2002). Molecular clock analyses estimate that the group M and O epidemics began in the early 20th century (Korber et al. 2000; Lemey et al. 2004; Worobey et al. 2008), while groups N and P appear to be more recent, though data is still limited.
Interestingly, despite eastern chimpanzees (P. t. schweinfurthii) also being widely infected with SIVcpzPts, there is no evidence of human transmission from this subspecies. Several factors might explain this: potentially lower human exposure to eastern chimpanzees, unrecognized human infections due to limited sampling, or differences in viral adaptation. SIVcpzPtt may have evolved mechanisms to overcome human immune barriers that SIVcpzPts lacks.
Unraveling the Origins of HIV-2
HIV-2, primarily confined to West Africa, exhibits a distinct epidemiological pattern compared to HIV-1. HIV-2 infections generally have lower viral loads and transmission rates, and disease progression is often slower (Popper et al. 2000; Berry et al. 2002; Rowland-Jones and Whittle 2007).
The origin of HIV-2 was traced back to sooty mangabeys (Cercocebus atys) and their simian immunodeficiency virus, SIVsmm (Hirsch et al. 1989; Gao et al. 1992; Chen et al. 1996). SIVsmm is highly prevalent and non-pathogenic in sooty mangabeys (Silvestri 2005; Santiago et al. 2005). Sooty mangabeys are frequently hunted as agricultural pests in West Africa, providing opportunities for human exposure and cross-species transmission.
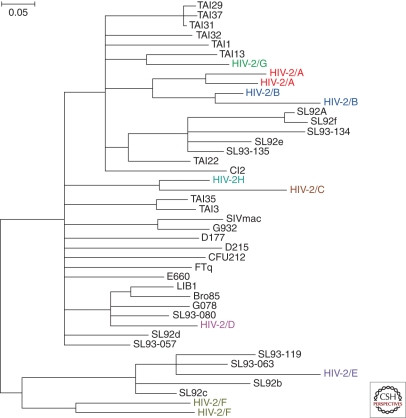
At least eight distinct HIV-2 lineages (groups A-H) have been identified, each representing an independent transmission from sooty mangabeys to humans (Fig. 5). Groups A and B are responsible for most human HIV-2 infections, while the other groups are rare, often found in single individuals, suggesting limited secondary spread (Damond et al. 2001; Peeters et al. 2003; Pieniazek et al. 1999; Ishikawa et al. 2001; Gao et al. 1992; Chen et al. 1996, 1997; Santiago et al. 2005). However, the recent identification of a second case of group F HIV-2 with signs of pathogenicity (Smith et al. 2008) raises questions about the potential for wider spread and evolution of less common HIV-2 groups.
 HIV-2 origins and related SIVsmm
HIV-2 origins and related SIVsmm
Figure 5. Phylogenetic tree showing HIV-2 and SIVsmm relationships. Eight HIV-2 groups, each from a separate sooty mangabey to human transmission, are highlighted. Groups A and B have spread more widely in humans.
Host-Specific Adaptations: Overcoming Immune Barriers
For a virus to successfully jump from one species to another and establish a new infection, it must overcome various host-specific barriers. These include differences in host proteins required for viral replication and, importantly, host restriction factors – components of the innate immune system that defend against viral infections (Fu et al. 2009; Ortiz et al. 2009; Malim and Emerman 2008; Neil and Bieniasz 2009; Kajaste-Rudnitski et al. 2010). Viruses, in turn, evolve counter-mechanisms to antagonize these restriction factors, often in a species-specific manner.
One example of host-specific adaptation in HIV-1 is a change in the viral matrix protein (Gag-30). A methionine (Met) residue conserved in ape SIVcpz and SIVgor changed to arginine (Arg) in the ancestors of HIV-1 groups M, N, and O upon transmission to humans (Wain et al. 2007). This specific amino acid substitution, occurring independently in multiple transmission events, suggests strong selective pressure in the human host. Reciprocal transmission experiments and in vitro studies confirmed the functional importance of this residue for viral replication in chimpanzee versus human cells (Mwaengo and Novembre 1998; Wain et al. 2007).
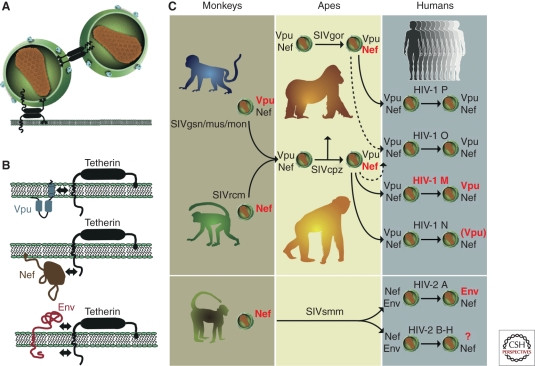
Host restriction factors like APOBEC3G, TRIM5α, and tetherin are crucial in shaping the success or failure of cross-species SIV transmission. Tetherin, in particular, appears to have played a significant role in the emergence of HIV-1 and HIV-2 (Fig. 6). Tetherin inhibits viral release from infected cells (Neil et al. 2008). SIVs often use their Nef protein to counteract tetherin in their natural hosts (Jia et al. 2009; Zhang et al. 2009). HIV-1, and some monkey SIVs, utilize Vpu protein to degrade tetherin (Neil et al. 2008; Van Damme et al. 2008; Sauter et al. 2009; Schmokel et al. 2011; Iwabu et al. 2009; Rong et al. 2009). Other viruses use their envelope glycoprotein (Env) to interfere with tetherin (Bour et al. 1996; Gupta et al. 2009; Le Tortorec and Neil 2009; Serra-Moreno et al. 2011).
 Tetherin function and viral antagonism
Tetherin function and viral antagonism
Figure 6. Tetherin and viral countermeasures. (A) Tetherin restricts virus release. (B) Viruses use different proteins (Vpu, Nef, Env) to antagonize tetherin. (C) HIV-1 and HIV-2 and their precursors evolved different strategies to overcome human tetherin, often involving adaptations in Vpu (HIV-1) or Env (HIV-2).
Human tetherin has a deletion in its cytoplasmic domain compared to other apes (Sauter et al. 2009). This deletion renders SIVcpz Nef ineffective against human tetherin. Gorilla tetherin lacks this deletion, so SIVgor Nef remains functional in gorillas (Fig. 6C). To replicate in humans, HIV-1 and HIV-2 precursors needed to evolve new mechanisms to counteract human tetherin. HIV-1 group M successfully regained Vpu-mediated anti-tetherin activity (Sauter et al. 2009), while other HIV-1 groups (O, P, N) show limited or compromised tetherin antagonism. HIV-2 group A adapted by using its Env protein to counteract tetherin (Le Tortorec and Neil 2009). This suggests that overcoming human tetherin was a critical step in the emergence of epidemic HIV strains.
The Origin of the AIDS Pandemic in Kinshasa
HIV-1’s rapid evolution, due to error-prone reverse transcriptase and short generation time (Li et al. 1988; Lemey et al. 2006; Ho et al. 1995; Wei et al. 1995), provides a molecular clock to trace the pandemic’s origins. Phylogenetic analyses date the most recent common ancestor of HIV-1 group M to between 1910 and 1930 (Korber et al. 2000; Worobey et al. 2008). This indicates that HIV-1 circulated undetected for decades before the AIDS pandemic was recognized in the 1980s.
Epidemiological and genetic evidence points to Kinshasa (formerly Leopoldville) in the Democratic Republic of Congo as the epicenter of the HIV-1 group M pandemic (Vidal et al. 2000; Zhu et al. 1998; Worobey et al. 2008). Early HIV-1 group M diversification occurred in Kinshasa, with all known subtypes and unique lineages identified there. Analysis of blood samples from Kinshasa in 1959 and 1960 revealed that HIV-1 had already diversified into different subtypes by that time (Worobey et al. 2008).
Demographic factors also support Kinshasa’s role. Early 20th-century Kinshasa experienced rapid urbanization, becoming the largest city in the region. River networks, major transportation routes at the time, connected the chimpanzee reservoir in southeastern Cameroon to Kinshasa on the Congo River (Sharp and Hahn 2008). The confluence of zoonotic introduction, urbanization, and connectivity facilitated the early spread and diversification of pandemic HIV-1 in Kinshasa, making it the likely “cradle of the AIDS pandemic.”
As HIV-1 group M spread globally, founder effects and migration patterns led to the geographic distribution of different subtypes and circulating recombinant forms (CRFs) (Taylor et al. 2008). Subtype B, dominant in Europe and the Americas, originated from a single strain that likely spread from Africa to Haiti in the 1960s and then to the US (Gilbert et al. 2007). CRF01, prevalent in Southeast Asia, likely emerged in Central Africa and spread through Thailand (Taylor et al. 2008). Emerging evidence suggests that different subtypes may have varying biological properties that influence their epidemiology and pathogenicity (Taylor et al. 2008; Kiwanuka et al. 2010; Sacktor et al. 2009).
Conclusion: Understanding Zoonotic Origins and Future Risks
Research into primate lentiviruses has revealed the complex origins and evolutionary pathways of HIV-1 and HIV-2. Where did AIDS originate from? The answer lies in the cross-species transmission of simian immunodeficiency viruses from non-human primates in Africa to humans. HIV-1 originated from SIVcpz in chimpanzees in Cameroon, while HIV-2 originated from SIVsmm in sooty mangabeys in West Africa.
Host restriction factors like tetherin play a crucial role in limiting cross-species transmission, and overcoming these barriers requires viral adaptation. The emergence of the AIDS pandemic was likely facilitated by a combination of factors, including zoonotic transmission, viral adaptation to overcome human immune defenses, urbanization, and social changes that promoted viral spread in early 20th-century Africa (Pepin et al. 2006, 2010; Pepin and Labbe 2008; Chitnis et al. 2000; Worobey et al. 2008; de Sousa et al. 2010).
Understanding the zoonotic origins of AIDS is critical for predicting and preventing future pandemics. Continued research into primate lentiviruses, host restriction factors, and the dynamics of cross-species transmission is essential for global health security. The story of AIDS serves as a stark reminder of the interconnectedness of human and animal health and the ongoing risk of emerging infectious diseases originating from the animal kingdom.
ACKNOWLEDGMENTS
We thank Lilian Pintea for ape range maps, Frank Kirchhoff for unpublished data, Gerald Learn for phylogenetic tree construction, and Jamie White for artwork and manuscript preparation. This work was supported by grants from the National Institutes of Health (R01 AI50529, R01 AI58715, P30 AI 27767), and the Bristol Myers Freedom to Discover Program.
REFERENCES
*Reference is also in this collection.
A006841C1 Aghokeng AF, et al. 2007. Origin of a new simian immunodeficiency virus in mandrills. J Virol 81: 9780–9793.
A006841C2 Aghokeng AF, et al. 2010. High prevalence and genetic diversity of simian immunodeficiency virus in wild-living mandrills across Gabon and Cameroon. J Virol 84: 7020–7032.
A006841C3 Apetrei C, et al. 2005. Tracing the origin and evolution of simian immunodeficiency virus SIVmac in macaques. J Virol 79: 11112–11124.
A006841C4 Apetrei C, et al. 2006. Molecular epidemiology of simian immunodeficiency virus SIVsm in naturally infected sooty mangabeys (Cercocebus atys) from Liberia. J Virol 80: 4909–4920.
A006841C5 Arhel N, Kirchhoff F. 2009. Nef-mediated downregulation of CD3: a conserved function in primate lentiviruses? Retrovirology 6: 104.
A006841C6 Bailes E, et al. 2003. Mosaic structure of simian immunodeficiency virus from chimpanzees. J Virol 77: 2707–2719.
A006841C7 Barouch DH. 2008. Challenges in the development of a preventive HIV-1 vaccine. Nature 455: 613–619.
A006841C8 Barre-Sinoussi F, et al. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220: 868–871.
A006841C9 Berry KM, et al. 2002. Low maternal viral load in human immunodeficiency virus type 2 (HIV-2)-infected women is associated with a low risk of vertical transmission. J Infect Dis 186: 561–565.
A006841C10 Bour S, et al. 1996. Envelope glycoprotein of human immunodeficiency virus type 1 induces receptor-mediated down-modulation of CD4 and major histocompatibility complex class I molecules. J Virol 70: 8172–8180.
A006841C11 CDC (Centers for Disease Control). 1981. Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep 30: 250–252.
A006841C12 Chakrabarti L, et al. 1987. Sequence organization of simian immunodeficiency virus from macaque and its relationship to human immunodeficiency virus. Nature 328: 543–547.
A006841C13 Chen Z, et al. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of novel HIV-2 strains from the eastern part of Sierra Leone. AIDS Res Hum Retroviruses 13: 745–754.
A006841C14 Chen Z, et al. 1996. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic origin of human immunodeficiency virus type 2. J Virol 70: 3617–3627.
A006841C15 Chitnis A, et al. 2000. Origin of the HIV-1 pandemic: assessment of iatrogenic versus non-iatrogenic scenarios. AIDS Res Hum Retroviruses 16: 1005–1015.
A006841C16 Clavel F, et al. 1986. Human retrovirus with envelope antigenically related to simian immunodeficiency virus. Science 233: 343–346.
A006841C17 Cohen MS, et al. 2011. Antiretroviral treatment prevents HIV-1 transmission. N Engl J Med 365: 493–505.
A006841C18 Damond F, et al. 2001. Diversity and intergroup recombination of human immunodeficiency virus type 2 in urban Guinea-Bissau. J Virol 75: 5955–5968.
A006841C19 De Leys RJ, et al. 1990. Isolation and partial characterization of an unusual human immunodeficiency retrovirus from two persons of west-central African origin. J Virol 64: 1207–1216.
A006841C20 de Silva E, et al. 2008. HIV-2 in West Africa: prevalence, incidence and clinical features. AIDS 22 (Suppl 3): S37–S45.
A006841C21 de Sousa JD, et al. 2010. Genital ulcers and the expansion of HIV-1 subtype C. PLoS ONE 5: e13451.
A006841C22 Etienne L, et al. 2011. Pathogenic SIVcpz infection in wild chimpanzees. PLoS Pathog 7: e1001311.
A006841C23 Evans DT, et al. 2010. Tetherin, virus release and virus countermeasures. Retrovirology 7: 87.
A006841C24 Fabre PH, et al. 2009. A glimpse into the pattern of macaque evolution. Mol Phylogenet Evol 53: 243–255.
A006841C25 Fischer A, et al. 2006. Genetic diversity and differentiation among chimpanzee populations in Central Africa. Curr Biol 16: 2204–2208.
A006841C26 Fu W, et al. 2009. Virus-host interactions: HIV-1 and the human proteome. Adv Virus Res 75: 297–322.
A006841C27 Fultz PN, et al. 1990. Prevalence of natural infection with simian immunodeficiency virus and human immunodeficiency virus type 2 in mandrills. AIDS Res Hum Retroviruses 6: 1359–1361.
A006841C28 Gagneux P, et al. 1999. Mitochondrial DNA sequences and the genetic origin of chimpanzee populations. Evolution 53: 458–468.
A006841C29 Gallo RC, et al. 1984. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224: 500–503.
A006841C30 Gao F, et al. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397: 436–441.
A006841C31 Gao F, et al. 1992. Evidence for widespread distribution of human immunodeficiency virus type 2 and related viruses in West Africa. Lancet 340: 861–864.
A006841C32 Gifford RJ, et al. 2008. Evolution and distribution of class II-related endogenous retroviruses in lemurs. J Virol 82: 6778–6786.
A006841C33 Gilbert C, et al. 2009. Independent invasions of the genome by endogenous lentiviruses in Strepsirrhine primates. J Virol 83: 676–687.
A006841C34 Gilbert MT, et al. 2007. The emergence of HIV-1 subtype B in the Americas. Nature 446: 381–385.
A006841C35 Gonder MK, et al. 2011. Genetic diversity and structure in chimpanzee populations: evidence from genome-wide microsatellite markers. PLoS Genet 7: e1002234.
A006841C36 Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Belknap Press of Harvard University Press, Cambridge, MA.
A006841C37 Gray RH, et al. 2001. Probability of heterosexual transmission of HIV-1 per coital act in rural Uganda. Lancet 357: 1049–1053.
A006841C38 Greene WC. 2007. AIDS and the immune system. Immunol Rev 219: 5–24.
A006841C39 Groves CP. 2001. Primate taxonomy. Smithsonian Institution Press, Washington, DC.
A006841C40 Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704.
A006841C41 Gupta RK, et al. 2009. HIV-1 Vpu and not Env is the primary tetherin antagonist in primary macrophages. PLoS Pathog 5: e1000650.
A006841C42 Gupta RK, Towers GJ. 2009. Primate antiviral restriction factors. Genome Biol 10: 237.
A006841C43 Gurtler LG, et al. 1994. HIV-1 group O in Germany. Lancet 344: 1443–1444.
A006841C44 Guyader M, et al. 1987. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature 326: 662–669.
A006841C45 Hahn BH, et al. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287: 607–614.
A006841C46 Hamel C, et al. 2007. Trends in HIV-1 and HIV-2 prevalences in the general population of rural Guinea-Bissau: evidence for a decline in HIV-2 prevalence. AIDS 21: 755–759.
A006841C47 Heeney JL, et al. 2006. The pathogenesis of HIV-1 and SIV infections. Nat Med 12: 410–423.
A006841C48 Hill CP, et al. 1996. Crystal structure of the HIV-1 matrix protein p17 at 1.7-A resolution. Proc Natl Acad Sci USA 93: 3099–3104.
A006841C49 Hirsch VM, et al. 1989. An African primate lentivirus related to HIV-2 and SIVmac. Nature 339: 389–392.
A006841C50 Hladik F, McElrath MJ. 2008. HIV-1 infection of human genital mucosa. Immunol Rev 223: 265–281.
A006841C51 Ho DD, et al. 1995. Rapid turnover of plasma virions and CD4+ T cells in HIV-1 infection. Nature 373: 123–126.
A006841C52 Holmes EC. 2003. Molecular clocks and the puzzle of primate lentivirus evolution. Philos Trans R Soc Lond B Biol Sci 358: 223–229.
A006841C53 Huet T, et al. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345: 356–359.
A006841C54 Ishikawa K, et al. 2001. High genetic diversity of HIV-2 group B in Cote d’Ivoire. AIDS Res Hum Retroviruses 17: 1439–1442.
A006841C55 Iwabu Y, et al. 2009. HIV-1 Vpu counteracts restriction by tetherin/BST-2 through disruption of trafficking. Genes Dev 23: 1522–1533.
A006841C56 Jia X, et al. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu against SERINC5 restricts cross-species transmission. Cell Host Microbe 6: 115–125.
A006841C57 Jin MJ, et al. 1994a. Analysis of sequence diversity in env gene of simian immunodeficiency viruses from African green monkeys: evidence for two genetically distinct virus subtypes. J Virol 68: 6253–6260.
A006841C58 Jin MJ, et al. 1994b. Identification of a highly divergent simian immunodeficiency virus SIVver from a vervet monkey: evidence for a new species-specific lineage. J Virol 68: 2754–2760.
A006841C59 Kajaste-Rudnitski A, et al. 2010. Antiviral restriction factors. J Mol Biol 403: 678–694.
A006841C60 Katzourakis A, et al. 2007. Endogenous lentiviruses are widespread in rabbits and hares. J Virol 81: 10706–10716.
A006841C61 Keele BF, et al. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with simian immunodeficiency virus. Nat Med 15: 465–470.
A006841C62 Keele BF, et al. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313: 523–526.
A006841C63 Kirchhoff F. 2009. Pathogenesis of HIV and SIV: are we any closer to understanding? Cell Host Microbe 6: 405–416.
A006841C64 Kirchhoff F. 2010. Antagonism of host restriction factors: HIV-1 versus SIV. Cell Host Microbe 8: 55–67.
A006841C65 Kiwanuka N, et al. 2010. HIV-1 subtype D is associated with more rapid disease progression than subtype A in Rakai, Uganda. AIDS 24: 1547–1552.
A006841C66 Klatt NR, et al. 2011. High prevalence of lentivirus infection in wild monkeys despite minimal MHC class I diversity. J Virol 85: 4971–4979.
A006841C67 Korber BT, et al. 2000. Timing the ancestor of the HIV-1 pandemic strains. Science 288: 1789–1796.
A006841C68 Le Tortorec A, Neil SJ. 2009. Antagonism of tetherin restriction of HIV-1 release by Vpu and Env. PLoS Pathog 5: e1000553.
A006841C69 Leendertz FH, et al. 2011. Assessing zoonotic disease risks at the human-wildlife interface: insights from primate lentivirus research. One Health 9: 7–17.
A006841C70 Lemey P, et al. 2004. Bayesian phylogeographic analysis of the origin and dispersion of human immunodeficiency virus type 1 group O. J Virol 78: 9537–9544.
A006841C71 Lemey P, et al. 2003. Tracing the origin and history of the HIV-2 epidemic. Proc Natl Acad Sci USA 100: 11008–11013.
A006841C72 Lemey P, et al. 2006. The molecular population genetics of HIV-1 revealed by analyzing the pattern of gaps in the genome. Genetics 173: 529–539.
A006841C73 Li WH, et al. 1988. Rates of mutation in human genes and retroviruses. Mol Biol Evol 5: 313–330.
A006841C74 Li Y, et al. 2010. Origin and dissemination of simian immunodeficiency virus in wild chimpanzees. J Virol 84: 6748–6757.
A006841C75 Lim ES, et al. 2010. Rapid evolution of functional antagonism between primate tetherin and lentiviral Vpu proteins. PLoS Pathog 6: e1000850.
A006841C76 Malim MH, Emerman M. 2008. HIV-1 sequence variation: drift, shift, and escape. Cell 135: 833–845.
A006841C77 Mauclere P, et al. 1997. Geographic distribution and demographic characteristics of HIV-1 group O infection in west central Africa. AIDS 11 (Suppl A): S17–S24.
A006841C78 McNatt MW, et al. 2009. Host-pathogen arms race: identification of positive selection in TRIM5alpha genes of African green monkeys. PLoS Pathog 5: e1000336.
A006841C79 Merson MH, et al. 2008. Global epidemiology of HIV-1. Lancet 372: 1601–1612.
A006841C80 Mitani JC, Watts DP. 1999. Why do chimpanzees hunt and share meat? Behav