Introduction
The human brain, a marvel of biological engineering, orchestrates a vast symphony of processes, from the simplest reflexes to the most intricate cognitive functions. Within its complex architecture, basal nuclei and structures of the limbic system stand out, playing pivotal roles in motor control, learning, and the intricate tapestry of memory 1. Maintaining cerebral homeostasis, the brain’s delicate equilibrium, hinges on a finely tuned interplay between a diverse population of neurons, glial cells, and non-neuronal cells. This balance is fundamentally rooted in precise gene regulation across all brain structures 2. Remarkably, approximately 30% of human genes are expressed predominantly within this vital organ. Furthermore, the level of gene transcription in the brain exhibits significant regional variability, with specific gene groups showing co-expression patterns in distinct brain areas 3. However, many studies have analyzed homogenized tissue, necessitating a deeper understanding of cellular heterogeneity and gene functionality within specific brain regions.
Disruptions in gene dosage can precipitate a spectrum of disorders, frequently manifesting as neurological dysfunctions. Down syndrome (DS), arising from a partial or complete triplication of genes on chromosome 21, represents the most common aneuploidy associated with varying degrees of cognitive impairment 4 – 5. Research into partial trisomies has delineated a region on chromosome 21, termed the Down Syndrome Critical Region (DSCR), located at the distal end of the long arm (21q22.1-22.3). This region harbors candidate genes whose imbalance may contribute to significant cognitive deficits and other associated pathologies 6.
While the DSCR’s exclusive role in DS symptomatology remains debated, evidence suggests its primary involvement in genetic interactions linked to DS pathogenesis 7. However, the precise subset of overexpressed genes on chromosome 21 causing these deficits remains incompletely understood 8, with some not exhibiting overexpression in trisomic fetal brains 9. Therefore, a detailed understanding of DSCR gene expression in normal human brains is crucial. Such knowledge can illuminate their roles in cognitive processes and shed light on their implications in neurological dysfunctions associated with DS and other neuropathologies, such as Alzheimer’s disease.
To investigate the cerebral transcriptome, methodologies like DNA microarrays have emerged, enabling the analysis of gene expression across numerous tissues. Comparative studies of expression profiles across brain regions have advanced our understanding of the physiopathology of various diseases 10 – 11. However, challenges persist, including the acquisition of sufficient brain samples from donors without neuropsychiatric or neuropathological histories, proper tissue extraction, and maintaining optimal RNA quality across all structures.
In this context, bioinformatics databases have become invaluable, providing comprehensive data on gene transcription levels in various brain structures. These databases leverage rigorous computational tools and data from DNA microarray experiments on postmortem human brains. The Allen Institute of Brain Science’s Allen Human Brain Atlas (http://www.alleninstitute.org) stands as a prominent public database. It encompasses anatomical measurements of gene expression and genomic information from human brains, offering visualization and data mining tools. With extensive coverage from histological preparations and microarray data (over 62,000 probes), it allows access to transcriptional data for thousands of genes referenced by Entrez Gene Encode. For 93% of the 21,245 genes listed, at least two different probes are available.
This study employs a bioinformatics model to analyze the expression profiles of 19 DSCR genes across 24 substructures of cerebral nuclei and 18 from the limbic lobe in normal human brains. Gene expression data, derived from DNA microarray experiments, were obtained from the freely accessible database of the Allen Institute for Brain Sciences’ Brain Atlas (http://www.brain-map.org). The findings contribute to a systemic expression model that can be bioinformatically adapted to explore other brain pathologies, offering a powerful tool for understanding brain function and disease.
Materials and Methods
Type of study
This research constitutes a descriptive bioinformatics study (in silico) utilizing the Allen Human Brain Atlas, a freely accessible online database (http://human.brain-map.org) supported by the Allen Institute for Brain Sciences. The study focused on the expression levels of 19 DSCR genes (21q22.12-22.2) within the cerebral nuclei and the limbic lobe.
Data mining
Using the graphic cursor on the Allen Institute for Brain Sciences’ human brain project webpage, gene expression level values and standard data (z-scores) were identified and recorded for various substructures of the limbic lobe and cerebral nuclei. These values were then compiled into Excel spreadsheets for subsequent analysis. For each substructure, three values were collected from different points, and the mean z-score was used as the representative data point. The z-scores were normalized values ranging from -3 to +3. Additional genomic information was extracted from the Gene Ontology (GO) application (http://www.geneontology.org/index.shtml). This included cellular location, molecular function, and biological processes associated with the selected genes.
Calculating the Z-score
To provide context for the z-score data, a general overview of the experimental procedures is warranted. Detailed procedures are available in the Allen Institute for Brain Science’s technical reports. Z-scores were calculated by subtracting the total average intensity of genes on the microarray from the intensity of each individual gene within a single experiment. This difference was then divided by the standard deviation (SD) of all intensity measurements on the microarray, according to the formula: Z score = (Gene intensity – average intensity of all genes) / SD of all gene intensities. Here, “Gene” refers to a specific gene on the microarray, and “all genes” represents the aggregate measure of all genes on the microarray 12.
Selection of genes and brain structures
The primary criterion for selecting the nineteen genes in this study was their product’s association with clinical manifestations of Down syndrome (Table 1). Cerebral nuclei and limbic lobe structures were chosen due to their higher expression levels observed in a preliminary study involving eight DSCR genes 13. Table 2 provides key information for each of the nineteen genes.
Table 1. Protein-coding genes at HSA21/DSCR in this study. Gene ID, name, and molecular process information from Gene Entrez database (http://www.ncbi.nlm.nih.gov/gene/).
Table 2. Structures of Limbic Lobe and Cerebral Nuclei with names from Allen Institute for Brain Sciences.
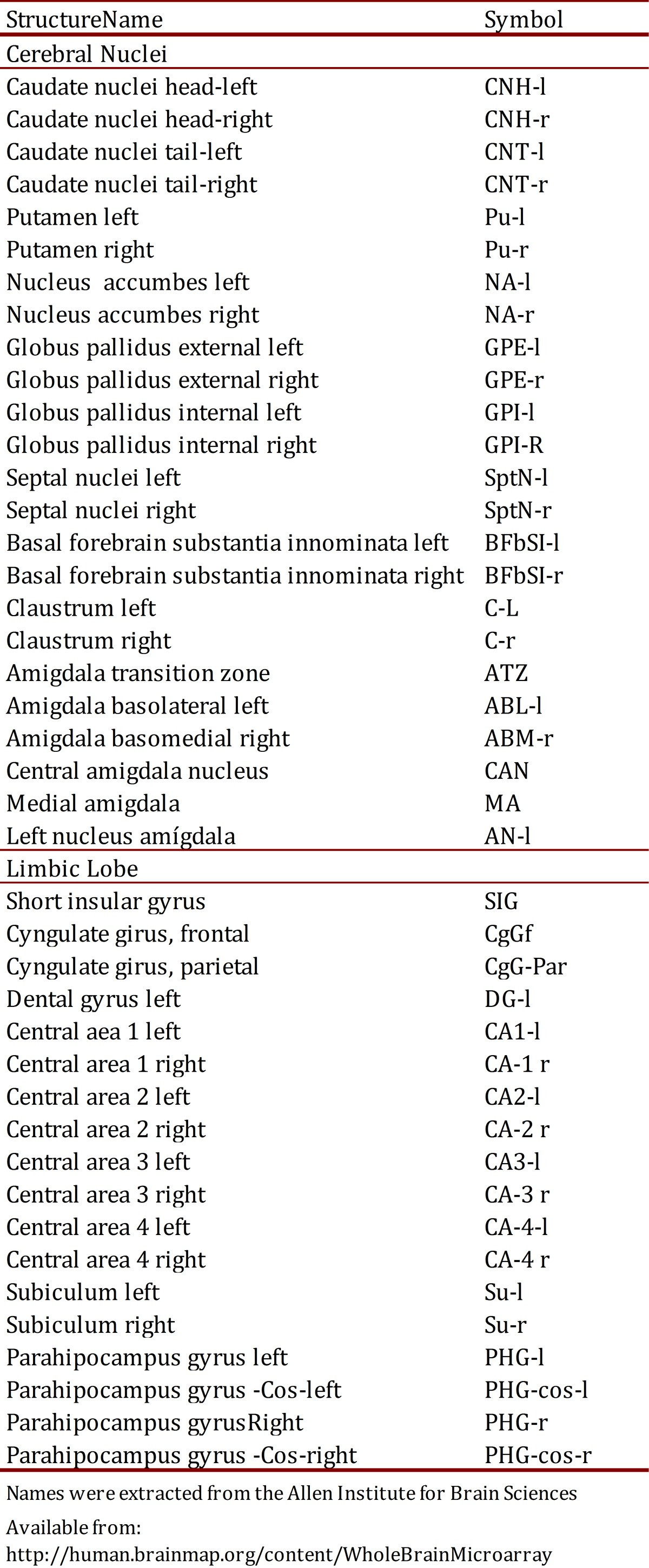 Table 2. Names and symbols of different structures of Limbic Lobe and Cerebral Nuclei. The names were extracted from the Allen Institute for Brain Sciences.
Table 2. Names and symbols of different structures of Limbic Lobe and Cerebral Nuclei. The names were extracted from the Allen Institute for Brain Sciences.
Statistical analysis
Statistical analyses were performed using STATGRAPHICS Centurion XVI (http://www.statgraphics.com) and Systat 13 (http://www.systat.com). Differences between data sets were assessed using multiple comparison analysis, with significance determined by the Mann-Whitney test for multiple comparisons. Scatter plots were used to model expression level differences per gene and anatomical area, using Pearson partial correlation values for comparison with a 95% significance level (p < 0.05, R > 0.6). Principal Component Analysis (PCA) was complemented by Cluster Analysis (CA).
Results
Differential expression of DSCR genes in the human brain
Quantitative analysis of global DSCR gene transcription across cerebral nuclei and limbic lobe structures revealed the highest z-scores in the putamen for DSCR3 and RCAN1. Elevated expression was also found in the left and right caudate nuclei for DSCR6, and in the left and right globus pallidus for DSCR3, SH3GBR, DYRK1A, CLIC6, and PRMT2. Within the limbic lobe, the dentate gyrus (DG) showed peak expression for DYRK1A and KCNJ6, while CA1 and CA2 regions exhibited highest expression for DSCR3 and KCNJ6 (Fig. 1A). Conversely, the lowest expression levels were observed in basal caudate nuclei for KCNJ6 and PSMG1, and in CA3 and CA4 for PIGP, DSCR6, and PRMT2 (Fig. 1B).
Figure 1. Global expression profiles of nineteen genes located at HAS21/DSCR across brain substructures of cerebral nuclei and limbic lobe. (A) Genes overexpressed per brain substructure. (B) Genes underexpressed per brain substructure. Mean z-scores from Allen Human Brain Atlas (http://www.alleninstitute.org) transcription data. Abbreviations: CNH-l, Caudate nuclei head-left; CNH-r, Caudate nuclei head-right; CNT-l, Caudate nuclei tail-left; CNT-r, Caudate nuclei tail-right; Pu-l, Putamen left; Pu-r, Putamen right; GPE-l, Globus pallidus external left; GPI, Globus pallidus internal; DG, Dental gyrus; CA1, Central area 1; CA2, Central area 2; CA3, Central area 3; CA-4, Central area 4.
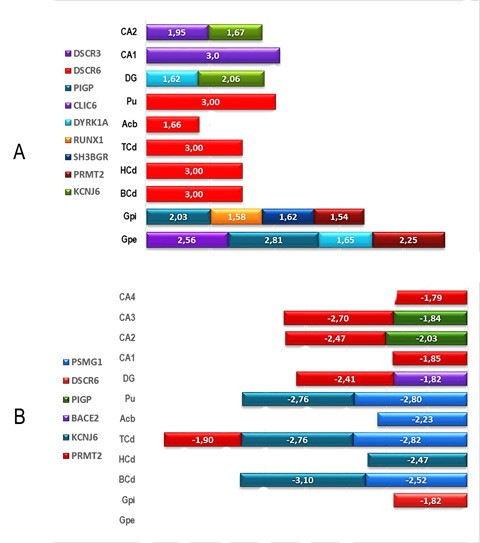 Figure 1. Global expression profiles of DSCR genes across brain regions, showing over and under expressed genes.
Figure 1. Global expression profiles of DSCR genes across brain regions, showing over and under expressed genes.
Expression in cerebral nuclei
The RCAN1 gene exhibited high expression in the left putamen (z-score 2.87), right putamen (2.66), and left external globus pallidus (2.67), while showing low expression in the left caudate nucleus (-1.268). DSCR2 was not overexpressed in any cerebral nuclei structures, but the left putamen, right putamen, and left external globus pallidus showed the lowest transcription levels among these structures. DSCR3 expression was generally low across cerebral nuclei, but the left external globus pallidus showed the highest z-score (1.089), contrasting with the left amygdala (-1.28).
DSCR4 showed high z-scores in the nucleus accumbens (1.84) and right putamen (1.40), but a low z-score in the left amygdala (-1.022). PIGP expression peaked in the right putamen (1.97). DSCR6 expression varied across cerebral nuclei, with highest z-scores in the left putamen (2.95), left caudate nucleus head (2.04), right caudate nucleus head (2.31), and right caudate nucleus tail (2.62). DSCR8’s highest z-score (1.46) was in the left putamen. DSCR10 showed high expression in the right caudate nucleus (2.58) and right external globus pallidus (2.16), but the lowest z-score for ATZ (-1.72).
BACE2 and ERG expression peaked in the left external and internal globus pallidus (2.44/2.09 and 2.37/2.22, respectively). BACE2 was also overexpressed in the right globus pallidus internal (2.176). DSCAM, DYRK1A, and KCNJ6 showed low expression in the caudate nucleus (-1.94/-1.86/-2.28). DYRK1A and KCNJ6 were also underexpressed in the right external globus pallidus (-1.69/-1.57). KCNJ6 also showed low expression in the left (-2.40) and right (-2.16) putamen and left internal and external globus pallidus (-1.78/-1.53). RUNX1 and SIM2 showed maximum underexpression in the amygdala nucleus (-1.93/-1.62). RUNX1 showed mid-range overexpression in caudate nucleus, putamen, and globus pallidus. BRWD1, CLIC6, ETS2, and SH3BGR generally exhibited low expression levels.
Expression in the limbic lobe
In the left CA4 region, DSCR1, 2, 3, 4, 5, 6, and 8 showed highest z-scores (2.04 ± 0.56). Variable expression levels were observed in other limbic lobe structures, with DSCR1 generally showing the lowest expression. PIGP expression peaked in the right CA4 region (2.09). DSCR6 showed low expression in the subiculum (-1.39). CLIC6 was highly expressed in the left CA1 region (2.807) and right parahippocampal gyrus (2.147). BRWD1 and DYRK1A showed low expression in CA4 (-1.598/-1.506, respectively). BACE2 and RUNX1 showed variably low expression in the left dentate gyrus (-2.431/-1.355, respectively). ERG, ETS2, SH3BGR, and SIM2 generally showed low expression levels.
Co-expression of genes located in DSCR across brain regions
Cluster Analysis (CA) using median distance values revealed co-expression patterns between certain genes and substructures in both cerebral nuclei and limbic lobe (Fig. 2). A strong co-expression association was found between DSCR2 and KCNJ6 in CA3 and dentate gyrus. Conversely, RUNX-1, ERG, and SIM2 showed high co-expression association in caudate nucleus and globus pallidus. In the caudate nucleus, close co-expression was observed for DSCR1, DSCR6, DCR10, and CLIC-6.
Figure 2. Heatmap of cluster analysis using median distance values of nineteen DSCR genes across substructures of Cerebral Nuclei and Limbic Lobe. Red indicates higher association, green indicates lower association.
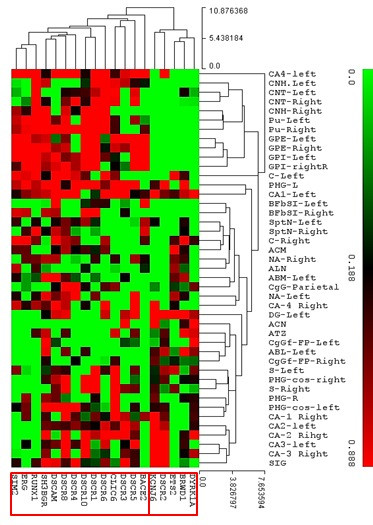 Figure 2. Heatmap visualizing cluster analysis of DSCR gene co-expression in brain regions.
Figure 2. Heatmap visualizing cluster analysis of DSCR gene co-expression in brain regions.
PCA for cerebral nuclei identified six components explaining 85.6% of total variance. The first component grouped DSCR1, 8, 10, BACE2, ERG, and RUNX1 genes, while the second component included DSCR4 and ETS2. For the limbic lobe, six components explained 76.1% of total variance. The first component grouped DSCR1, ERG, and RUNX1, while the second component grouped DSCR4, DSCR 8, and ETS2.
Biplots revealed two associations for cerebral nuclei: DSCAM, DSCR4, DSCR8, and SIM2 in the first, and ERG and RUNX in the second (Fig. 3 right). For the limbic lobe, two associations were also found: DSCR2, 5, and 6 in the first, and DSCAM and DSCR4 in the second (Fig. 3 left).
Figure 3. Distribution of nineteen DSCR genes in biplots from principal component analysis of expression values in limbic lobe (left) and cerebral nuclei (right).
Description: Two biplots resulting from principal component analysis of gene expression values. The left biplot represents the limbic lobe, and the right biplot represents the cerebral nuclei, showing the distribution of 19 DSCR genes in a two-dimensional space defined by principal components.
Discussion
The Human Genome Project’s mid-term impact includes the vast data repositories now available, enabling bioinformatics analyses. These analyses offer new perspectives on biological phenomena in silico using genomic variables from online databases. This study leveraged such resources to create a topographical map of 19 DSCR gene expression across normal human brain structures. The results demonstrate differential DSCR gene transcription dependent on the brain structure. Thus, in a normal brain, the analyzed DSCR genes exhibit precise transcriptional regulation varying across functional components.
Previous studies showed high RCAN1 expression in the cortex, midbrain, and cerebellum of mouse DS models 14, and PIGP gene expression in the developing brain 15. PIG-P, encoding a component of glycosylphosphatidylinositol-N-acetylglucosaminyltransferase (GPI-GnT), was overexpressed in the cerebral cortex, while DSCR6 expression was normal. DSCR4 was not detected in the fetal brain 16. While these studies indicated structure-specific differential transcription, our findings provide a more detailed view of DSCR gene transcription, showing gene-specific profiles dependent on brain substructure.
Our results showed RCAN1 and RUNX1 overexpression in brain structures linked to learning and cognition, contrasting with DYRK1A underexpression in the same regions. RUNX1 protein, a heterodimeric transcription factor binding to enhancer and promoter core elements, is involved in normal hematopoiesis 17. Chromosomal translocations involving RUNX1 are associated with leukemias 18.
RCAN1, encoding a protein interacting with calcineurin A and inhibiting calcineurin-dependent signaling, potentially affects CNS development 19. Located in the minimal candidate region for Down syndrome phenotype, RCAN1 is overexpressed in DS fetal brains. Chronic overexpression may contribute to neurofibrillary tangles, as seen in Alzheimer’s disease 20.
DYRKA1, part of the dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) family, may regulate cell proliferation and brain development. It is considered a strong candidate gene for learning defects in Down syndrome 21. DYRK1A phosphorylates transcription factors like CREB and NFAT, endocytic proteins, and AD-linked gene products. RCAN1, an endogenous calcineurin A inhibitor, contributes to learning and memory deficits, altered synaptic plasticity, cell cycle dysregulation, and Alzheimer’s-like neuropathology in DS 22. This suggests a potential feedback mechanism in transcriptional control within these brain areas.
A key finding was the differential transcriptional control via negative feedback for several genes depending on brain substructure. For example, RCAN1 and RUNX1 overexpression contrasted with DYRK1A underexpression in caudate nuclei, putamen, and globus pallidus of normal brains. This suggests that in normal brains, gene expression in cerebral nuclei and limbic lobe structures regulates cognitive and emotional functions through a complex gene expression network responsible for learning, memory, and related processes 23 – 24. DYRK1A overexpression, contrasting with underexpression in normal brains in our study, is implicated in deregulation of multiple pathways in developing and aging DS brains, causing structural and functional alterations, including mental retardation and dementia. Enhanced amyloid precursor protein phosphorylation by overexpressed DYRK1A promotes amyloidogenic cleavage, increasing Aβ40 and leading to brain β-amyloidosis in Alzheimer’s disease 25.
The putamen, part of the basal nuclei, is crucial in operative conditioning (reinforcement learning) 26. Projections from somatosensory and motor cortices, thalamic intralaminar nucleus, and substantia nigra project to the putamen, which then projects to motor and premotor cortex areas via the globus pallidus and thalamus 27. The caudate nucleus, another basal ganglia component, is involved in movement modulation and learning and memory, particularly feedback processing. The limbic lobe, including the cingulate gyrus, subcallosal gyrus, and parahippocampal gyrus, is central to emotional life and memory formation, interacting with the hypothalamus, hippocampus, amygdala, and other brain areas. The high transcription levels of certain DSCR genes in these structures may correlate with their roles in cognitive and emotional functions 28 , 29.
Calcineurin-nuclear factor of activated T cells (NFAT) signaling is critical in immune, nervous, and cardiovascular systems, and in endothelial cell activation during angiogenesis or inflammation 30. A 1.5-fold increase in DSCR1 and DYRK1A dosage destabilizes a regulatory circuit, reducing NFATc activity and contributing to Down syndrome features. Mathematical modeling suggests autoregulation within the DSCR1, DYRK1A, and NFATc pathway amplifies trisomy effects, impairing target gene activation under specific conditions 31.
Brain homeostasis relies on complex gene interactions within a diverse cellular environment, ultimately stemming from precise gene regulation. This study confirms and expands this concept, proposing a systemic model of global gene expression for 19 DSCR genes in key normal human brain structures that control learning, movement, and emotions.
Acknowledgments
We thank Universidad del Valle and Universidad Autónoma de Occidente for financial support and research infrastructure.
References
[1] (B01) Nieuwenhuys R, Voogd J, Huijzen C van. Das zentrale Nervensystem des Menschen: ein morphologischer Atlas. Berlin: Springer; 1988.
[2] (B02) Sutcliffe JS, Nurmi EL, Bell JT, Singhmar P, পেক্মেয্চি B, পেক্মেয্চি M, et al. Association analysis of common SNPs across 1.1 million human gene-centric tags implicates novel genes for autism spectrum disorders. Mol Psychiatry. 2009;14(6):585–94.
[3] (B03) Cahoy JD,

