The electron transport chain (ETC) is a fundamental process in cellular respiration, acting as the cell’s powerhouse for energy generation. Understanding where electron transport occurs is crucial to grasping how life sustains itself at a microscopic level. This article delves into the precise locations of the ETC in different types of organisms, elucidating its components and significance in energy production.
Understanding the Electron Transport Chain
What is the Electron Transport Chain?
The electron transport chain (ETC) is a series of protein complexes embedded in a membrane that facilitates a sequence of oxidation-reduction reactions. This chain is essential for generating the majority of ATP (adenosine triphosphate), the primary energy currency of the cell, through a process called oxidative phosphorylation. Essentially, the ETC harnesses the energy from electron transfer to pump protons across the membrane, creating an electrochemical gradient. This gradient then drives the synthesis of ATP.
Key Components of the ETC
The ETC is composed of several key components:
- Electron Carriers: These molecules, including cytochromes, flavoproteins, iron-sulfur proteins, and quinones, are responsible for shuttling electrons along the chain. Their varying redox potentials ensure a stepwise transfer of electrons.
- Protein Complexes: In eukaryotes, these carriers are organized into four major protein complexes (Complex I-IV) within the inner mitochondrial membrane. Prokaryotes have analogous complexes located in their plasma membrane, often with more diversity in carrier types.
- ATP Synthase (Complex V): This enzyme is the culmination of the ETC process. It utilizes the proton gradient generated by the electron transport chain to phosphorylate ADP (adenosine diphosphate) into ATP.
- Electron Donors and Acceptors: The ETC starts with electrons donated from molecules like NADH and FADH2, derived from earlier stages of cellular respiration (like the Krebs cycle). It terminates with a final electron acceptor, typically oxygen in aerobic respiration, but can be other substances in anaerobic respiration.
Location of Electron Transport Chain: A Comparative View
The location of electron transport varies between eukaryotes and prokaryotes, reflecting their cellular structures.
Electron Transport Chain Location in Eukaryotes: Mitochondria
In eukaryotic cells, the electron transport chain is located in the inner mitochondrial membrane. Mitochondria are often referred to as the “powerhouses of the cell,” and the ETC is a central reason for this designation.
- Inner Mitochondrial Membrane: This membrane is highly folded into cristae, increasing the surface area available for ETC complexes. The complexes (I-IV) are embedded within this membrane, along with mobile carriers like coenzyme Q and cytochrome c.
- Intermembrane Space: As electrons move through the ETC, protons (H+) are pumped from the mitochondrial matrix into the intermembrane space. This creates a proton gradient across the inner mitochondrial membrane.
- ATP Synthase Location: ATP synthase (Complex V) is also located in the inner mitochondrial membrane, allowing it to utilize the proton gradient to produce ATP within the mitochondrial matrix.
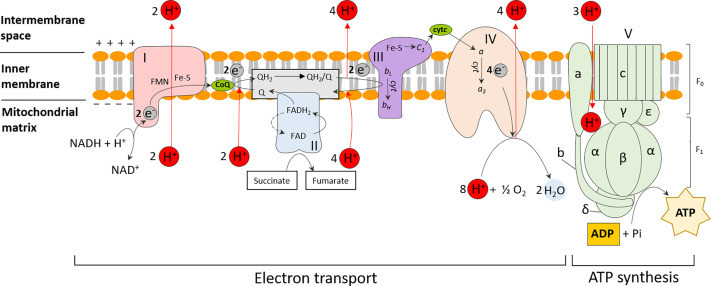 Eukaryotic Electron Transport Chain Location: Detailed diagram of oxidative phosphorylation in the inner mitochondrial membrane, showing Complexes I-V, Coenzyme Q (CoQ), Cytochrome c (CytC), and F0F1 ATP synthase for ATP production during cellular respiration.
Eukaryotic Electron Transport Chain Location: Detailed diagram of oxidative phosphorylation in the inner mitochondrial membrane, showing Complexes I-V, Coenzyme Q (CoQ), Cytochrome c (CytC), and F0F1 ATP synthase for ATP production during cellular respiration.
Fig. 1. Oxidative phosphorylation in eukaryotes. ATP synthesis is coupled to the sequential transfer of electrons via an electron transport chain from NADH or FADH2 to oxygen. This process is facilitated by a series of electron carriers, which also serve to establish the proton motive force by translocating protons outside the membrane. This generates an electrochemical gradient which fuels the final ATP synthesis step. Abbreviations are as follows: complex I, NADH coenzyme Q reductase; complex II, succinate dehydrogenase; complex III, cytochrome bc1; complex IV, cytochrome c oxidase; complex V, F0F1 ATP synthase; CoQ, coenzyme Q; cytC, cytochrome c. The subunits (a, b, c, α, β, δ, ε and γ) which make up the F0 and F1 units of complex V are also indicated.
Electron Transport Chain Location in Prokaryotes: Plasma Membrane
In prokaryotic cells (bacteria and archaea), which lack mitochondria, the electron transport chain is located in the plasma membrane (also known as the cell membrane).
- Plasma Membrane as ETC Site: The plasma membrane of prokaryotes serves as the analogous location to the inner mitochondrial membrane in eukaryotes. The ETC complexes and electron carriers are embedded within this membrane.
- Periplasmic Space: In bacteria, the proton gradient is established across the plasma membrane, with protons being pumped from the cytoplasm into the periplasmic space (the region between the plasma membrane and the outer membrane in Gram-negative bacteria). In archaea and Gram-positive bacteria, the protons are pumped outside the cell.
- ATP Synthase in Plasma Membrane: Similar to eukaryotes, ATP synthase is also located in the plasma membrane of prokaryotes, utilizing the proton gradient to synthesize ATP within the cytoplasm.
The Process of Electron Transport and Energy Generation
Regardless of whether it’s in the mitochondria or the plasma membrane, the fundamental process of electron transport remains similar.
Electron Flow and Energy Generation
Electrons, carried by molecules like NADH and FADH2, enter the ETC at different complexes. As electrons move through the chain, they pass from carriers with lower electron affinity to those with higher affinity, releasing energy at each step. This energy is used by Complexes I, III, and IV in eukaryotes (and analogous complexes in prokaryotes) to pump protons across the membrane.
The sequential transfer of electrons is crucial because it allows for a controlled release of energy, preventing damage to the cell. This stepwise energy release is efficiently coupled to proton pumping, establishing the electrochemical gradient, also known as the proton-motive force.
Role of ATP Synthase in Oxidative Phosphorylation
The electrochemical gradient created by proton pumping is a form of potential energy. ATP synthase (Complex V) acts like a molecular turbine. As protons flow back down their electrochemical gradient, through ATP synthase and into the mitochondrial matrix (in eukaryotes) or cytoplasm (in prokaryotes), this flow of protons drives the rotation of a part of the enzyme. This mechanical rotation is then converted into chemical energy as ATP synthase catalyzes the phosphorylation of ADP to ATP. This process is termed chemiosmosis, where the movement of ions across a semipermeable membrane, down their electrochemical gradient, is coupled to the generation of ATP.
Inhibitors and Uncouplers of the Electron Transport Chain
The ETC is a tightly regulated and essential process. Certain substances can disrupt its function:
- Inhibitors: Substances like cyanide and carbon monoxide can block electron flow within the ETC, typically by binding to specific complexes (e.g., Complex IV). This halts proton pumping and ATP synthesis.
- Uncouplers: Uncouplers, such as dinitrophenol (DNP), disrupt the coupling between electron transport and ATP synthesis. They allow protons to leak across the membrane, bypassing ATP synthase. While electron transport may continue, the proton gradient is not maintained, and ATP is not efficiently produced. The energy is released as heat instead.
Conclusion: The Critical Location for Life’s Energy
In summary, electron transport occurs at the inner mitochondrial membrane in eukaryotes and the plasma membrane in prokaryotes. This precise localization is vital for creating the proton gradient necessary for ATP synthesis through oxidative phosphorylation. The ETC’s location within these membranes allows for the compartmentalization of the process, ensuring efficient energy conversion and highlighting the elegance and efficiency of cellular respiration in sustaining life across all domains.

