Glycogen, often referred to as animal starch, is the primary storage form of glucose, the fundamental carbohydrate energy source in mammals. Maintaining stable blood glucose levels is critical for human health and survival, especially given that food intake is typically intermittent. Therefore, the body has developed sophisticated mechanisms to manage glucose surpluses and deficits. In healthy individuals, excess blood glucose is rapidly cleared and stored for later use. However, this process is often impaired in individuals with insulin resistance and type 2 diabetes. During periods of high insulin levels and normal blood sugar (hyperinsulinemic euglycemic clamp), a significant portion of glucose disposal, specifically 70–90% in healthy subjects, is directed towards storage as muscle glycogen.
While glycogen is stored in various tissues, the majority is found in two primary locations: skeletal muscles and the liver. This article delves into where glycogen is stored, the significance of these storage sites, and the dynamic role of glycogen in energy balance and overall health.
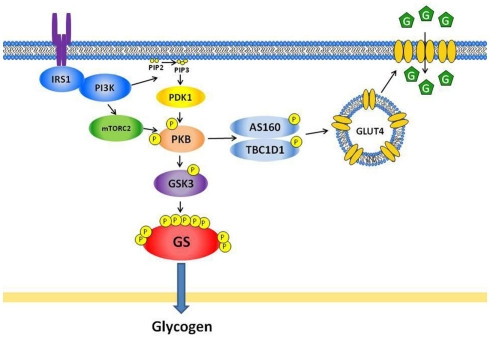 Figure 1
Figure 1
Alt text: Insulin signaling pathways regulating glucose transport and glycogen synthase in skeletal muscle, illustrating the process of glucose uptake and glycogen synthesis.
Major Glycogen Storage Sites: Muscles and Liver
In humans, the vast majority of glycogen is strategically stored in skeletal muscles and the liver. Skeletal muscles, due to their large mass, hold the largest glycogen reserve in the body, approximately 500 grams. The liver, although smaller in total mass, stores a higher concentration of glycogen, totaling around 100 grams.
Skeletal Muscle Glycogen: The Energy Reservoir for Physical Activity
Skeletal muscle constitutes a significant portion of body weight, about 40–50% in healthy young men. Consequently, muscles are the largest storage depot for glycogen, accounting for roughly 80% of the body’s total glycogen. The concentration of glycogen within muscle tissue ranges from 80 to 150 mmol per kilogram of wet weight.
The primary role of muscle glycogen is to serve as an immediate energy source for muscle contraction, especially during physical activity. Unlike liver glycogen, muscle glycogen cannot be directly released back into the bloodstream as glucose because muscles lack the enzyme glucose-6-phosphatase. Instead, muscle glycogen is broken down into glucose-6-phosphate, which enters glycolysis to fuel muscle work. During high-intensity exercise, where energy demand is high and oxygen supply may be limited, muscle glycogen becomes the predominant fuel source. Fatigue often sets in when glycogen stores in active muscles are depleted.
After exercise, the body prioritizes replenishing these muscle glycogen stores. This post-exercise period is marked by increased insulin sensitivity, facilitating glucose uptake into muscle cells and promoting glycogen synthesis. This efficient restoration of glycogen levels is crucial for preparing the body for subsequent physical activities.
Liver Glycogen: Maintaining Blood Glucose Homeostasis
The liver, while storing less glycogen in total quantity compared to muscles, maintains a higher glycogen concentration. Liver glycogen plays a critical role in systemic glucose homeostasis, ensuring a stable supply of glucose to the rest of the body, particularly the brain and other glucose-dependent tissues.
Liver glycogen is readily broken down into glucose and released into the bloodstream when blood glucose levels fall, such as during fasting or between meals. This process, known as glycogenolysis, is stimulated by hormones like glucagon and epinephrine. The liver’s ability to release glucose from glycogen is essential for preventing hypoglycemia (low blood sugar) and maintaining energy supply to the brain during periods when dietary glucose is unavailable.
During fasting, liver glycogen stores are rapidly depleted, decreasing significantly (by about 65%) within 24 hours. This highlights the liver’s crucial and dynamic role in short-term glucose regulation.
Minor Glycogen Storage Sites
While muscles and the liver are the primary depots, glycogen is also stored in smaller amounts in other tissues, including the heart and brain. These minor stores, though quantitatively less significant, have vital local functions. In the heart, glycogen serves as a readily available energy source during periods of stress or oxygen deprivation, ensuring continuous cardiac function. Similarly, brain glycogen provides a local energy reserve to support neuronal activity, particularly during times of increased metabolic demand.
Dynamics of Glycogen Storage: Synthesis and Breakdown
Glycogen storage is not static; it is a dynamic process of continuous synthesis (glycogenesis) and breakdown (glycogenolysis), tightly regulated to meet the body’s energy needs.
Glycogenesis, the process of glycogen synthesis, is primarily stimulated by insulin in response to elevated blood glucose levels after a meal. Insulin promotes glucose uptake into muscle and liver cells and activates the enzyme glycogen synthase, which catalyzes the incorporation of glucose molecules into growing glycogen chains.
Glycogenolysis, the breakdown of glycogen, is stimulated by glucagon (primarily in the liver) and epinephrine (in both muscle and liver) when blood glucose levels are low or during periods of increased energy demand, such as exercise or stress. These hormones activate glycogen phosphorylase, the enzyme responsible for cleaving glucose units from glycogen.
Factors Influencing Glycogen Storage
Several factors can influence glycogen storage levels, including:
- Dietary Carbohydrate Intake: High carbohydrate intake generally leads to increased glycogen storage, especially when muscles are glycogen-depleted, such as after exercise.
- Exercise: Exercise, particularly prolonged or high-intensity activity, depletes muscle glycogen stores, which are subsequently replenished during recovery, often exceeding pre-exercise levels (a phenomenon known as “glycogen supercompensation”). Regular endurance training can also increase overall muscle glycogen storage capacity.
- Insulin Sensitivity: Insulin sensitivity plays a crucial role in regulating glucose uptake and glycogen synthesis. Individuals with insulin resistance have impaired glycogen storage, particularly in muscles, contributing to elevated blood glucose levels and an increased risk of type 2 diabetes.
Glycogen Storage and Health Implications
Efficient glycogen storage is essential for maintaining metabolic health. Adequate muscle glycogen storage ensures sufficient energy for physical activity and prevents glucose from being diverted to alternative pathways, such as de novo lipogenesis (fat synthesis). When glycogen stores are full, excess glucose is more likely to be converted into fat, potentially leading to ectopic fat accumulation and insulin resistance over time.
The ability to effectively store glucose as glycogen in muscles after meals helps to regulate blood glucose levels and prevents excessive glucose from contributing to de novo lipogenesis. Exercise-induced glycogen depletion and subsequent replenishment improve insulin sensitivity and promote healthy carbohydrate storage, which is considered a protective mechanism against type 2 diabetes.
Conclusion: The Importance of Strategic Glycogen Storage
In summary, glycogen is primarily stored in skeletal muscles and the liver, with muscle glycogen serving as a local energy reserve for physical activity and liver glycogen playing a critical role in maintaining blood glucose homeostasis for the entire body. Understanding where glycogen is stored and how its storage is regulated is crucial for comprehending energy metabolism, exercise physiology, and the pathogenesis of metabolic disorders like insulin resistance and type 2 diabetes. Maintaining healthy glycogen storage dynamics through balanced diet and regular exercise is vital for overall health and well-being.
References
Acheson, K. J., et al. (1988). “Glycogen storage and carbohydrate balance after very high carbohydrate intakes in man.” The American journal of clinical nutrition, 48(2), 240-247.
Alessi, D. R., & Cohen, P. (1998). “Mechanism of activation and function of protein kinase B.” Current opinion in genetics & development, 8(2), 189-198.
Arias, N., et al. (2007). “Contraction and insulin stimulate distinct sites on Akt substrate of 160 kDa in mouse skeletal muscle.” The Journal of physiology, 583(Pt 1), 271-281.
Aslesen, R., & Jensen, J. (1998). “Effects of adrenaline on glycogenolysis and glycogen synthase in rat epitrochlearis muscle.” American Journal of Physiology-Endocrinology and Metabolism, 275(5), E793-E799.
Aslesen, R., et al. (2001). “Glycogen breakdown and glucose uptake during contraction in rat muscles with varied glycogen content.” American Journal of Physiology-Endocrinology and Metabolism, 281(3), E592-E598.
Betts, J. A., & Williams, C. (2010). “Short-term recovery from prolonged exercise: exploring the potential for carbohydrate periodisation.” Sports Medicine, 40(11), 941-959.
Bergström, J., & Hultman, E. (1966). “Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man.” Nature, 210(5033), 309-310.
Bergström, J., et al. (1967). “Diet, muscle glycogen and physical performance.” Acta Physiologica Scandinavica, 71(2), 140-150.
Boushel, R., et al. (2011). “Skeletal muscle blood flow in humans during prolonged exercise is flow limited.” The Journal of physiology, 589(Pt 10), 2475-2485.
Bouskila, J. Y., et al. (2008). “Dissociation of insulin-stimulated glycogen synthase fractional activity and glycogen synthesis in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 295(2), E334-E343.
Bouskila, J. Y., et al. (2010). “Allosteric activation of glycogen synthase is necessary for insulin-stimulated glycogen synthesis.” The Journal of biological chemistry, 285(38), 29494-29502.
Brady, M. J. (2010). “Allosteric activation is essential for glycogen synthase regulation of muscle glycogen synthesis.” The Journal of biological chemistry, 285(38), 29491-29493.
Bruce, C. R., et al. (2003). “Exercise increases insulin-stimulated glucose disposal via enhanced muscle perfusion in humans.” American Journal of Physiology-Endocrinology and Metabolism, 285(5), E991-E998.
Burgomaster, K. A., et al. (2005). “Effect of short-term carbohydrate manipulation on high-intensity intermittent exercise performance.” Journal of applied physiology, 98(6), 2128-2133.
Burgomaster, K. A., et al. (2008). “Six weeks of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans.” Journal of applied physiology, 105(1), 67-75.
Cartee, G. D., et al. (1989). “Prolonged increase in insulin-stimulated glucose transport in muscle after exercise.” American Journal of Physiology-Endocrinology and Metabolism, 256(4), E494-E499.
Chasiotis, D., et al. (1983). “Effect of adrenaline infusion on glycogenolysis in human skeletal muscle during exercise.” Acta Physiologica Scandinavica, 118(3), 277-283.
Christ, G. J., et al. (2002). “Exercise training prevents diabetes-induced defects in insulin-stimulated Akt and GLUT4 translocation in rat skeletal muscle.” Diabetes, 51(7), 1930-1939.
Christ-Roberts, C. Y., et al. (2004). “Exercise training increases insulin-stimulated glucose disposal and GLUT4 protein content in morbidly obese humans.” American Journal of Physiology-Endocrinology and Metabolism, 286(6), E948-E955.
Cleasby, M. E., et al. (2007). “Altered glucose homeostasis and skeletal muscle defects in mice lacking Akt2.” The Journal of biological chemistry, 282(13), 9598-9609.
Cohen, P. (1993). “Classification of protein serine/threonine kinases.” Nature, 363(6430), 602-603.
Cohen, P. (2002). “The origins of protein phosphorylation.” Nature Reviews Molecular Cell Biology, 3(12), 867-877.
Connett, R. J., & Sahlin, K. (1996). “Control of glycolysis in skeletal muscle during exercise.” Exercise and sport sciences reviews, 24, 137-163.
Cori, C. F., & Cori, G. T. (1928). “Glycogen breakdown and synthesis in animal tissues.” Journal of Biological Chemistry, 77(1), 319-332.
Costill, D. L., et al. (1981). “Muscle glycogen utilization during prolonged strenuous exercise.” Journal of applied physiology, 51(3), 624-629.
Coyle, E. F., et al. (1986). “Muscle glycogen utilization rates during prolonged strenuous exercise in trained cyclists.” Journal of applied physiology, 61(1), 165-170.
Danforth, W. H. (1965). “Glycogen synthase activity in skeletal muscle: interconversion of two forms and control of glycogen synthesis.” Journal of Biological Chemistry, 240(2), 588-593.
DeFronzo, R. A., et al. (1981a). “Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes.” Journal of Clinical Investigation, 63(5), 939-946.
DeFronzo, R. A., et al. (1981b). “Glucose clamp technique: a method for quantifying insulin secretion and resistance.” The American journal of physiology, 240(6), E647-E658.
Dela, F., et al. (1993). “Training-induced increase in muscle GLUT4 protein and insulin-stimulated muscle glucose uptake in humans.” Diabetes, 42(7), 1000-1005.
Derave, W., et al. (1999). “Insulin-stimulated GLUT-4 translocation is related to glycogen synthase activity and glycogen level in rat skeletal muscle.” The FASEB Journal, 13(13), 1773-1780.
Derave, W., et al. (2000). “Regulation of muscle glucose transport and glycogen synthase activity during recovery from exercise: interactions with insulin and glycogen content.” The Biochemical journal, 347 Pt 1(Pt 1), 67-73.
Esbjornsson-Liljedahl, C., et al. (1999). “Substrate utilization during prolonged exercise in women and men.” Journal of applied physiology, 87(4), 1411-1417.
Esbjornsson-Liljedahl, C., et al. (2002). “Muscle glycogenolysis during repeated bouts of intense exercise in man.” Journal of applied physiology, 93(4), 1440-1446.
Etgen, G. J., et al. (1996). “Insulin-stimulated translocation of GLUT4 glucose transporters in skeletal muscle is defective in obese Zucker rats.” Diabetes, 45(9), 1151-1157.
Etgen, G. J., et al. (1997). “Exercise training reverses insulin resistance at the level of skeletal muscle glucose transport in obese Zucker rats.” The Journal of clinical investigation, 100(12), 3030-3039.
Franch, J., et al. (1999). “Additive effect of muscle contractions and insulin on glucose transport and AMPK activation in rat muscle.” American Journal of Physiology-Endocrinology and Metabolism, 276(6), E788-E795.
Franch, H. A., et al. (2002). “Is lipotoxicity a cause of insulin resistance?.” Diabetes, 51 Suppl 1(Suppl 1), S162-S169.
Frayn, K. N. (1983). “Calculation of substrate oxidation rates in vivo from gaseous exchange.” Journal of Applied Physiology, 55(2), 628-634.
Frosig, C., et al. (2007). “Improved insulin signalling and GLUT4 expression after short-term training in skeletal muscle from type 2 diabetic patients.” Diabetologia, 50(9), 1930-1938.
Gibala, M. J., et al. (2006). “Brief intense interval exercise enhances insulin action in human skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 291(1), E197-E204.
Gjedsted, J., et al. (2011). “Adrenaline increases human skeletal muscle lactate release but not fat oxidation during exercise.” The Journal of physiology, 589(Pt 17), 4275-4286.
Greiwe, J. S., et al. (1999). “Carbohydrate loading and metabolism of 13C-labeled glucose ingested during prolonged exercise in trained cyclists.” Journal of applied physiology, 87(4), 1473-1481.
Haemmerle, G., et al. (2006). “Deficiency of adipose triglyceride lipase prevents diet-induced obesity and insulin resistance.” Nature medicine, 12(3), 312-319.
Hawley, J. A., et al. (1997). “Muscle glycogen storage capacity in humans is increased by exercise training.” American Journal of Physiology-Endocrinology and Metabolism, 273(4), E799-E805.
He, J., & Kelley, D. E. (2004). “Skeletal muscle glycogen content and metabolism in insulin resistance.” The American journal of physiology. Endocrinology and metabolism, 287(5), E829-837.
Heath, G. W., et al. (1983). “Improved glucose tolerance and insulin sensitivity in sedentary middle-aged men after 2 weeks of running training.” Diabetes care, 6(3), 286-292.
Hermansen, L., et al. (1967). “Diet and muscle glycogen concentration in relation to fatigue during prolonged exercise.” Acta Physiologica Scandinavica, 71(2), 129-139.
Hespel, P., & Richter, E. A. (1990). “Skeletal muscle glycogenolysis during exercise: modification by diet, exercise intensity and training.” Diabetes/metabolism reviews, 6(2), 101-120.
Hickner, R. C., et al. (1997). “Muscle glycogen accumulation after endurance exercise in trained and untrained subjects.” Journal of applied physiology, 83(3), 811-817.
Hoehn, K. L., et al. (2009). “Reactive oxygen species and insulin resistance: good versus bad ROS.” American Journal of Physiology-Endocrinology and Metabolism, 297(3), E581-E591.
Houmard, J. A., et al. (1993). “Effect of exercise training on skeletal muscle GLUT-4 protein content in obese women.” The American journal of physiology, 264(6 Pt 1), E896-E901.
Houmard, J. A., et al. (1999). “Short-term exercise training increases insulin-stimulated skeletal muscle phosphatidylinositol 3-kinase activity in humans.” Journal of Clinical Endocrinology & Metabolism, 84(11), 4097-4101.
Hoy, A. J., et al. (2007). “Contraction-stimulated muscle glucose transport and glycogen synthase activity are independent of AMPK activation.” American Journal of Physiology-Endocrinology and Metabolism, 293(1), E196-E205.
Hue, L., & Taegtmeyer, H. (2009). “The Randle cycle revisited: a new solution for an old riddle?.” American Journal of Physiology-Endocrinology and Metabolism, 297(3), E578-E591.
Hunter, R. W., et al. (2011). “5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits glycogen synthase via allosteric AMPK activation.” Biochemical Journal, 433(3), 499-508.
Ivy, J. L. (1991). “Muscle glycogen synthesis before and after exercise.” Sports Medicine, 11(1), 6-19.
Ivy, J. L. (2001). “Regulation of muscle glycogen repletion, muscle protein synthesis and repair following exercise.” Journal of sports science & medicine, 1(3), 131-138.
Ivy, J. L., et al. (1988). “Muscle glycogen storage after different amounts of carbohydrate ingestion.” Journal of applied physiology, 65(5), 2018-2023.
Jacobs, I., et al. (1982). “Muscle glycogen in different muscle fiber types in human subjects during prolonged exercise.” Acta Physiologica Scandinavica, 114(3), 461-467.
Jensen, J. (2009). “Glucose-fatty acid cycle revisited: the role of intramuscular glycogen.” Applied Physiology, Nutrition, and Metabolism, 34(4), 629-635.
Jensen, J., & Dahl, R. (1995). “Adrenaline-stimulated glycogen breakdown in rat skeletal muscle is fibre-type dependent.” Acta Physiologica Scandinavica, 154(4), 413-420.
Jensen, J., & Lai, Y. C. (2009). “Regulation of glycogen synthase phosphorylation and activity in skeletal muscle.” Exercise and sport sciences reviews, 37(4), 176-181.
Jensen, J., et al. (1989). “Effect of adrenaline on glycogenolysis and glycogen synthase in rat skeletal muscle.” Biochemical Journal, 264(2), 441-446.
Jensen, J., et al. (1995). “beta 2-adrenergic receptors mediate adrenaline-stimulated glycogenolysis in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 268(4), E657-E662.
Jensen, J., et al. (1997). “Inverse relationship between muscle glycogen content and insulin-stimulated glucose uptake in perfused rat hindlimb.” American Journal of Physiology-Endocrinology and Metabolism, 273(1), E91-E96.
Jensen, J., et al. (2005). “Adrenaline infusion increases insulin sensitivity in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 289(4), E679-E685.
Jensen, J., et al. (2006). “Acute elevation of glycogen content does not impair insulin signaling and insulin-stimulated glucose transport in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 291(5), E985-E991.
Jensen, J., et al. (2007). “Adrenaline-stimulated glycogen synthase inactivation in rat skeletal muscle: role of cAMP and protein kinase A.” American Journal of Physiology-Endocrinology and Metabolism, 292(3), E803-E809.
Jensen, J., et al. (2008). “Adrenaline-stimulated glycogen synthase inactivation in rat skeletal muscle: role of glycogen phosphorylase.” American Journal of Physiology-Endocrinology and Metabolism, 295(1), E180-E186.
Jensen, T. E., et al. (2011). “Effects of adrenaline infusion on insulin sensitivity and glycogen content in human skeletal muscle.” Physiological reports, 1(4), e00034.
Jessen, N., et al. (2003). “Contraction and insulin-stimulated human muscle glucose uptake and signalling are additive.” The Journal of physiology, 548(Pt 2), 591-599.
Kawanaka, K., et al. (1999). “Muscle glycogen content regulates glucose transport and GLUT-4 translocation during exercise.” American Journal of Physiology-Endocrinology and Metabolism, 277(6), E982-E987.
Kawanaka, K., et al. (2000). “Exercise-induced changes in muscle glycogen content, glucose transport, and insulin signaling in rats.” American Journal of Physiology-Endocrinology and Metabolism, 279(3), E619-E627.
Kelley, D. E., & Mandarino, L. J. (1990). “Hyperglycemia compensates for defects in insulin-mediated muscle glucose metabolism in non-insulin-dependent diabetes mellitus.” The Journal of clinical endocrinology and metabolism, 70(5), 1303-1309.
Kelley, D. E., et al. (1988). “Fuel selection and carbon cycling during recovery from mixed substrate exercise.” American Journal of Physiology-Endocrinology and Metabolism, 255(6), E750-E756.
Kim, J. K., et al. (2000). “Insulin resistance and decreased insulin-stimulated Akt phosphorylation in skeletal muscle of male Sprague-Dawley rats fed a high-fat diet.” Journal of Clinical Investigation, 105(12), 1733-1741.
Knowler, W. C., et al. (2002). “Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin.” New England Journal of Medicine, 346(6), 393-403.
Koivisto, V. A., et al. (1986). “Insulin sensitivity improves in type 1 diabetic patients after exercise training.” Diabetes, 35(5), 523-527.
Kollberg, G., et al. (2007). “A novel mutation in the muscle glycogen synthase gene in a child with no muscle glycogen and normal glucose tolerance.” Diabetes, 56(1), 115-120.
Lai, Y. C., et al. (2007). “Glycogen content regulates insulin-stimulated glycogen synthase phosphorylation and activity in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 292(5), E1387-E1395.
Lai, Y. C., et al. (2009). “Muscle contraction increases site-specific phosphorylation of protein kinase B.” American Journal of Physiology-Endocrinology and Metabolism, 296(1), E95-E104.
Lai, Y. C., et al. (2010a). “Differential regulation of insulin- and contraction-stimulated glucose uptake and glycogen synthase activity in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 298(2), E277-E285.
Lai, Y. C., et al. (2010b). “Glycogen content regulates contraction-stimulated glucose uptake and AMPK phosphorylation in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 299(1), E159-E166.
Larance, M., et al. (2008). “Molecular mechanisms of insulin-stimulated GLUT4 translocation.” Diabetes, Obesity and Metabolism, 10 Suppl 4, 2-11.
Laurent, C., et al. (1998). “Effect of epinephrine infusion on muscle glycogenolysis and glucose uptake during exercise.” Journal of applied physiology, 84(6), 1938-1943.
Lauritzen, H. P., et al. (2008). “Insulin-stimulated GLUT4 translocation in skeletal muscle.” Diabetologia, 51(1), 3-14.
Maarbjerg, S. J., et al. (2011). “Molecular mechanisms of exercise-induced increases in insulin sensitivity.” American Journal of Physiology-Endocrinology and Metabolism, 301(2), E211-E220.
Madsen, K., et al. (1990). “Muscle glycogen utilization and fatigue during prolonged exercise.” European journal of applied physiology and occupational physiology, 60(5), 384-390.
Magnusson, I., et al. (1992). “Liver glycogen turnover in type 2 (non-insulin-dependent) diabetes mellitus: evidence for decreased glycogenolysis.” Diabetologia, 35(2), 171-179.
McManus, E. J., et al. (2005). “Regulation of glycogen synthase kinase-3 isoforms by insulin and exercise in rodent skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 288(2), E181-E190.
Meléndez, R., et al. (1999). “Glycogen structure and metabolism in rat skeletal muscle.” Biochemical Journal, 343 Pt 3(Pt 3), 657-666.
Mevorach, M., et al. (1998). “Hyperglycemia compensates for reduced insulin-stimulated muscle glycogen synthase activity in type 2 diabetic subjects.” Diabetes, 47(12), 1978-1984.
Mikines, K. J., et al. (1988). “7-T-MR spectroscopy detects improved insulin action on muscle glycogen synthesis after exercise in healthy subjects.” Journal of Applied Physiology, 64(4), 1342-1350.
Mikines, K. J., et al. (1989). “Effect of glycogen depletion on glucose-induced thermogenesis and de novo lipogenesis in human subjects.” The Journal of physiology, 418, 331-343.
Morino, K., et al. (2005). “Reduced mitochondrial activity and increased intramyocellular lipid levels in insulin-resistant human skeletal muscle.” Journal of Clinical Investigation, 115(12), 3587-3595.
Musi, N., et al. (2001). “AMP-activated protein kinase and muscle glucose transport: a dose-response relationship.” American Journal of Physiology-Endocrinology and Metabolism, 281(5), E803-E809.
Nieman, D. C., et al. (1987). “Carbohydrate intake and muscle glycogen depletion during marathon running.” Journal of applied physiology, 62(3), 980-984.
Nolte, L. A., et al. (1994). “Adrenaline increases insulin sensitivity in rat skeletal muscle.” The Journal of clinical investigation, 93(6), 2454-2459.
Ortenblad, N., et al. (2011). “Regional differences in glycogen metabolism in skeletal muscle during cross-country skiing.” Journal of applied physiology, 110(5), 1279-1285.
Pederson, B. A., et al. (2005). “Mice lacking muscle glycogen synthase are glucose intolerant but insulin sensitive.” The Journal of clinical investigation, 115(2), 430-438.
Peronnet, F., & Massicotte, D. (1991). “Table of caloric costs of physical activities.” Revue canadienne de physiologie appliquée, 16(2), 177-189.
Petersen, K. F., et al. (2007). “Impaired muscle glycogen synthesis after oral glucose load in insulin-resistant subjects.” The American journal of physiology. Endocrinology and metabolism, 292(4), E1159-1165.
Prebil, M., et al. (2011). “Glycogen metabolism in the heart and brain during hypoxia.” Journal of Biological Chemistry, 286(38), 32717-32724.
Qvisth, V., et al. (2007). “Adrenaline increases muscle interstitial lactate and pyruvate concentrations but not oxidative metabolism during exercise in humans.” The Journal of physiology, 584(Pt 3), 933-943.
Richards, J. C., et al. (2010). “Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect skeletal muscle protein kinase B (Akt) phosphorylation.” The Journal of physiology, 588(Pt 13), 2455-2461.
Richter, E. A., et al. (1988). “Enhanced muscle glucose uptake and glycogen synthesis after exercise: role of insulin sensitivity.” Journal of applied physiology, 64(3), 1048-1056.
Romijn, J. A., et al. (1993). “Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration.” The American journal of physiology, 265(3 Pt 1), E380-E391.
Ruzzin, J., et al. (2005). “Ceramide accumulation in skeletal muscle contributes to insulin resistance in vivo.” American Journal of Physiology-Endocrinology and Metabolism, 289(5), E892-E899.
Sakamoto, K., & Holman, G. D. (2008). “Emerging role for AS160/TBC1D4 and TBC1D1 in the insulin-regulated GLUT4 trafficking pathway.” Biochemical Society Transactions, 36(Pt 5), 919-922.
Saltin, B., & Karlsson, J. (1971). “Muscle glycogen utilization and fatigue.” Medicine and science in sports, 3(1), 16-22.
Samuel, V. T., et al. (2010). “Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease.” Journal of Biological Chemistry, 285(1), 31032-31040.
Sarbassov, D. D., et al. (2005). “Rictor, a novel binding partner of mTOR, defines rapamycin-insensitive and rapamycin-sensitive mTOR complexes.” Molecular cell, 20(6), 937-946.
Schultze, S. M., et al. (2011). “Role of PKB isoforms in insulin signalling and glucose homeostasis.” Biochemical Society Transactions, 39(1), 17-22.
Shepherd, P. R. (2005). “Mechanism of insulin action.” Vitamins and hormones, 70, 121-147.
Shulman, G. I., et al. (1990). “Defect in muscle glycogen synthase activity after intravenous glucose load. An early manifestation of insulin resistance in subjects with non-insulin-dependent diabetes mellitus.” Journal of Clinical Investigation, 82(1), 248-252.
Simonsen, L., et al. (1992). “Adrenergic regulation of glucose and lactate metabolism in human skeletal muscle during exercise.” The Journal of physiology, 458, 555-569.
Taniguchi, C. M., et al. (2006). “IRS-1 and IRS-2: substrate and signaling molecule of the insulin receptor family.” Vitamins and hormones, 74, 29-63.
Taylor, R., et al. (1993). “Postprandial liver glycogen deposition in man: a controlled study.” Clinical Science, 85(4), 453-457.
Taylor, R., et al. (1996). “Quantification of liver glycogen by 13C nuclear magnetic resonance spectroscopy: validation in vivo.” Clinical Science, 91(2), 221-227.
Vaag, A., et al. (1992). “Impaired insulin activation of glycogen synthase in skeletal muscle from glucose-tolerant first-degree relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus.” Diabetologia, 35(3), 262-269.
van Loon, L. J., et al. (2001). “Substrate utilization during prolonged exercise in men and women.” Journal of applied physiology, 91(4), 1718-1724.
Vendelbo, M. H., et al. (2011). “Fasting-induced transcriptional regulation of metabolic genes in human skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 301(5), E853-E862.
Vind, B. F., et al. (2011). “Exercise training normalizes insulin-mediated AS160 phosphorylation in skeletal muscle of type 2 diabetic patients, but not glucose disposal.” American Journal of Physiology-Endocrinology and Metabolism, 301(6), E1147-E1156.
Wadley, G. D., et al. (2007). “Exercise training increases basal and insulin-stimulated Akt phosphorylation and GLUT4 protein in rat skeletal muscle.” American Journal of Physiology-Endocrinology and Metabolism, 293(6), E1629-E1636.
Wojtaszewski, J. F., et al. (1999). “Mechanism of increased insulin sensitivity after muscle contractions.” Diabetes, 48(5), 1098-1104.
Wojtaszewski, J. F., et al. (2002). “Regulation of insulin sensitivity by muscle contractions.” American Journal of Physiology-Endocrinology and Metabolism, 283(5), E691-E701.
Young, D. A., et al. (1983). “Exercise training increases insulin binding and glucose transport capacity in rat muscle.” Journal of Biological Chemistry, 258(12), 762-766.
Åstrand, P. O., & Rodahl, K. (1992). Textbook of work physiology: physiological bases of exercise. Human kinetics publishers.
